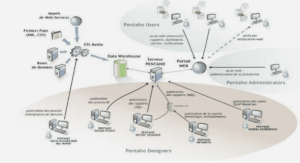
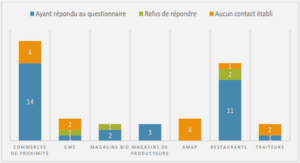
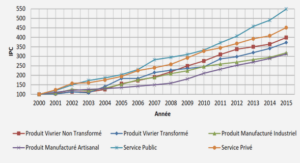
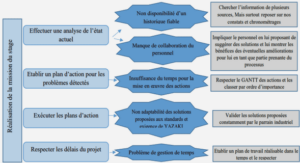
TRANSPORT OF HEAVY METALS IN MATERIALS WITH DIAMETER ANALOGOUS TO XYLEM VESSELS
TRANSPORT OF HEAVY METALS IN MATERIALS WITH DIAMETER ANALOGOUS TO XYLEM VESSELS:
In vascular plants, the soil solution is transported from the roots to the leaves through small diameter vessels found in the xylem; this transport not only allows the nutrient uptake but also the accumulation of heavy metals in their shoots. By analogy to this phenomenon, the present study aimed at the evaluation of the transport of heavy metals (Pb, Cr, As, Cd, Zn, Ni and Al) in solutions of pH 4 and 8 using a capillary siphon consisting of small pore diameter materials. Determination of the metal concentration in the solutions was performed by means of Inductively Coupled Plasma. The largest transport of metal ions is produced at pH 4, on ascending order: Cr<Pb=Al<As<Ni<Zn<Cd. Results showed that there is capillary transport of aqueous solutions with heavy metals in materials with diameter similar to that found in the xylem of plants. Some authors have argued that there is a remarkable similarity between soil and xylem, which makes possible the the soil-plant-atmosphere continuity; in this sense, the performance of the tested materials should be investigated under similar conditions to those present in the interface soil-xylem to attempt to replicate this continuity.
Soil pollution by toxic metals (Pb, Cr, As, Zn, Ni, Cd, etc.) is a growing preoccupation in the world that strongly affects industrialized countries (Belluck, 2006; Blais, 2010; Dermont et al., 2008; Sun et al., 2007). Given that heavy metals are not biologically degraded, the main problem with this type of elements stems from their mobility and their capacity of bioaccumulation in living organisms, starting with vegetables, which are placed at the beginning of the food chain (Garbisu and Alkorta, 2001; He et al., 2005; Pilon-Smits and Freeman, 2006).
The bioavailability of heavy metals is determined by their redistribution between the solid and aqueous phases of the soil. Because vegetable roots take up only the elements in the soil solution, the content of heavy metals in the aqueous phase of soils is essential in the soilplant transfer (Bourrelier et al., 1998; Fritioff and Greger, 2003; Girard et al., 2005).
A fundamental factor for heavy metal solubilization is pH (Ghorbani, 2008; Ghosh and Singh 2005b; Kabata-Pendias and Pendias, 2001). In soils, the pH range is commonly found between 3.5 and 9 (USDA, 1998) and, generally, low pHs increases the bioavailability of metals (Alloway and Jackson, 1991; Kashem and Singh, 2001; Rachou and Sauvé, 2008; Sinha et al., 2007). Thus, the transfer of metals towards plants has been described qualitatively as high for Zn, Ni and Cd and medium for Cr, Pb and As, in acidic conditions, while Pb, Cd, Zn and As exhibit low transfer and Cr, Ni and Al very low transfer, in basic conditions (Tremel-Shaub and Feix, 2005).
For the plant, heavy metals bioavailability is closely related to their solubility in the aqueous phase. Usually, soluble compounds are formed at low pH soil solution; as result of this, metal bioavailability increases for plants, and, on the contrary, fewer soluble compounds are formed at high pH soil solution, which reduces the heavy metal uptake by plants (Seregin and Ivanov, 2001. Table 1.6 exhibits the predominant heavy metals chemical species in soils and some aspects of mobility and bioavailability related to plants.
Determination of heavy metals:
Determination of the heavy metal concentration in the inlet and outlet solutions was made by ICP-OES Varian Vista MPX model. The calibration curve was obtained with a correlation coefficient of 0.99. The wavelengths (λ) used for the metals were: lead, λ=220,353 nm; nickel, λ=231,604 nm; zinc, λ=213,857 nm; arsenic, λ=228,812 nm aluminum, λ=396,152 nm; chrome, λ=267,716 nm and cadmium, λ=226,502 nm.
Statistical analysis :
Data obtained in this work were subjected to statistical analysis of variance (ANOVA) with the aid of JMP 8 software (SAS Institute Inc.). Differences were considered significant at P<0.05 using the Student’s t test. The Student’s t-test is a statistical significance test used for comparing the means of two treatments based on small independent samples (Caprette, 2013). A confidence level of 95% (P<0.05) is generally used in the field of chemical analysis (Efstathiou, 2013).
Comparison to hyperaccumulator plants :
Values of heavy metal content in plants considered as levels of accumulation and hyperaccumulation are presented in Table 2.2. These values were compared to those obtained for the tested materials in the present work, considering 1 kg of material. Because most of the hyperaccumulator plants are selective, criteria for defining a hyperaccumulator species are given as a function of single metals. Table 2.2 allows bringing into comparison the values of each metal transported and the sum of transport (T) and accumulation (A), as well as the total sum values (including all the metals present in the solution). In Table 2.2’s last row, values highlighted in bolds indicate that the tested materials showed similar levels to those reported individually for most metals in hyperaccumulator plants. These results exhibited M2 as more efficient to transport heavy metals and M1 with a greater accumulation capacity, with important correspondence to that reported for the translocation of metals in plants.
Heavy metals mobility and pH in soils :
As a general rule, mobility of heavy metals in soils is strongly related to pH conditions. Usually, acidic pH allows the mobilization of metals, while basic pH reduces the solubility of metals and, in consequence, their mobilization. Using a qualitative scale for the mobility of metals in soils at pH 4, the following behavior can be stated: Cd has strong mobility, Ni and Zn have medium mobility and Pb, Cr and Al have weak mobility (Kabata-Pendias and Pendias, 2001). Cd is reported as a very mobile metal in soils and readily available for plants, while Pb has low water solubility, which is why this metal turns out to be very immobile in soils (Miclean et al., 2000). In this sense, M1 displayed a great similarity to the described behavior.
HEAVY METAL TRANSPORT AS AN ANALOGY TO THE TRANSPIRATION PHENOMENON IN VASCULAR PLANTS :
Soil and water pollution with heavy metals is a growing concern in the world because of the risks associated to their mobility and bioaccumulation. Lead (Pb), arsenic (As), chromium (Cr), copper (Cu), nickel (Ni) and zinc (Zn) are the most commonly found toxic metals with a long residence time in polluted sites (Anawar et al., 2008; Blais et al., 2010; Jarup, 2003). In soils, mobility and bioavailability of heavy metals is strongly correlated to pH , this factor is very important for the soil-plant metal transfer. Usually, a low pH facilitates the metal uptake by plants (Fritioff and Greger, 2003; Ghorbani, 2008; Kabata Pendias and Pendias, 2001; Tremel-Schaub and Feix, 2005; Peijnenburg, 2004). The content of heavy metal in the soil solution of non-polluted soils ranges from 1 to 100 μg⋅L 1; accordingly, these values can be much higher in contaminated sites (Kabata-Pendias, 2004). The pollutant, which is bioavailable for plants in the aqueous phase of the soil (Krishnamurti and Naidu, 2007; Ying et al., 2002), reaches the roots by mass flow and diffusion phenomena (Tremel-Schaub and Feix, 2005) and passes through the root. From there, the soil solution rises through the xylem by capillary action and transpiration phenomenon, the latter of which is created by the pressure gradient between the root and the leaves (Campbell and Reece, 2004; Nijsee, 2004) that produces a suction effect, which allows the transport of solution in the vessels of the xylem (10-200 μm diameter) of vascular plants (Sperry et al., 2003).
Metal hyperaccumulator plants :
Depending on the species and the environmental conditions, some plants are able to take up considerable amounts of metals in their different aerial parts, such as leaves, stems, fruits and seeds (Cuningham et al., 1995; Glick, 2003; Krämer, 2005; Singh et al., 2007). Plants classified as hyperaccumulators are capable to transport and accumulate in their tissues amounts of a single heavy metal between 10 to 100 times more than those tolerated by crop plants (Kirkham, 2006; Kukier et al., 2004).
The rates of transport of hyperacumulator plants are a key factor in the implementation of plant-based methods for soil remediation allowing the removal of the contaminant from the site by harvesting the aerial parts of the plant. However, this type of species has certain disadvantages, such as slow growth, small biomass production, selective extraction, species that only can be used in their natural habitat among others (Kammev and Van der Lelie, 2000). In this context, the main objective of this study was the assessment of heavy metals transport from aqueous solutions at pH 4 and pH 8 using materials with pore diameter size in the range of xylem vessels at different suction pressures. The results were compared to reported values for hyperaccumulator plants to contrast the performances.
Pressure assumption:
Considering that the suction pressure estimated for trees to rise the soil solution 1 m is about 0.02 MPa·m-1 (Taiz and Zeiger, 2010), a proportional pressure was taken in the experiments to create a similar effect to the transpiration phenomenon in the xylem of vascular plants, that is 0.0067 MPa for a 30 cm displacement of the solutions containing the metal ions (Figure 4.1b). To evaluate the transport of metal ions in M1 and M2, 250 mL of solutions at pH 4 and pH 8 were suctioned at the above-mentioned pressure.
A conceptual model of heavy metal transport in the soil-material continuum :
By analogy to some features observed in the xylem of vascular plants, the model was developed considering i) the transport of solutions in a porous material M1 (biomaterial composed by cellulose) featuring capillary transport of heavy metals; ii) the simulation of a negative pressure effect of the same magnitude of that reported for the xylem of plants (-0.02 MPa·m-1); and iii) a coefficient of transport (TCHM) depending on the type of material, pH and transported metal ion, which was estimated based on similar criteria to that used to identify phytoaccumulator species of heavy metals.
Bioarrangements used to prevent soil pollution:
The use of biomaterials of low cost should be considered as an option since such materials are seen as wastes in various agricultural and food processes and are generated in large quantities. In the developed model, pressure conditions similar to those reported for the xylem and a cylinder arrange for the material tested were used. However, in Article 1, the capillary properties of the materials showed that the movement through the pore spaces could result in transport or accumulation of heavy metals on the material surface depending on the conditions of pH, metal in solution and material. Given these characteristics, other arrangements, like those disclosed by the nature, should be tried, such as several forms of tubers and roots. These can be tested as preventive methods of soil contamination, by adding biomaterials on the surface, based on forms similar to those of carrots, beets, potatoes or a tangle of roots, which can be retrieved later and taken to disposal or undergo desorption processes for recovering the heavy metal, regenerating also the material for re-use. In this way, the modification of biomaterials, or of the characterization of these, can result in the application of more suitable biomaterials for the type of metal present in the site of interest, the pH soil conditions and the type of active site in the material: carboxyl (R-COO-), hydroxyl (-OH), sulphate (R-OSO3-) and amino groups (R2-NH2 and R-NH).
CONCLUSION :
Nowadays, the ability of hyperaccumulator plants to extract and concentrate heavy metals in their aerial parts is a subject of great interest because its application in soil and water bioremediation puts forward alternatives to preserve these natural resource characteristics diminishing remediation costs in comparison to traditional methods. However, phytoremediation of heavy metals still has many challenges to overcome in order to be applied on-site with satisfactory results.
|
Table des matières
INTRODUCTION
CHAPTER 1 LITERATURE REVIEW
Heavy metal pollution and environmental problems
Methods to remove and stabilize heavy metals in soils
Phytoremediation .
Categories and application of phytoremediation
Accumulator and hyperaccumulator plants
Enrichment coefficient to identify hyperaccumulator species
Transfer of heavy metals to plants and pH conditions
Soil solution transport in vascular plants .
CHAPTER 2 TRANSPORT OF HEAVY METALS IN MATERIALS WITH DIAMETER ANALOGOUS TO XYLEM VESSELS
Introduction
Materials and methods
Building of the capillary syphon
Transport of heavy metals
Determination of heavy metals
Statistical analysis
Results and discussion
Volumes of solution transported by the materials
Heavy metals mobility in acidic conditions
Heavy metals mobility in basic conditions
Comparison to hyperaccumulator plants
Heavy metals mobility and pH in soils
Conclusions
CHAPTER 3 HEAVY METAL TRANSPORT AS AN ANALOGY TO THE TRANSPIRATION PHENOMENON IN VASCULAR PLANTS
Introduction
Pressure gradient in the xylem
Metal hyperaccumulator plants
Materials and methods
Capillary means of transport
Heavy metal solutions
Assessed pressures
Determination of heavy metals
Statistical analysis
Results and discussion
Effect of pH on the transport of heavy metals
Pressure effect
Metals in the soil-plant system
Hyperaccumulation criteria
Mobility of heavy metals in the soil-plant system
Conclusions
References
CHAPTER 4 TRANSPORT COEFFICIENT FOR HEAVY METALS IN POROUS MATERIALS
Introduction
Materials and methods
Capillary means of transport
Heavy metal solutions
Pressure assumption
Heavy metal determination
Calculation of transport coefficient of heavy metals (TCHM)
Statistical analysis
Results and discussion
Transport, accumulation and TCHM in acidic conditions
Transport, accumulation and TCHM in basic conditions
Transport Coefficient of heavy metals (TCHM) and Enrichment Coefficient (EC)
Conclusions
References
CHAPTER 5 DISCUSSION
A conceptual model of heavy metal transport in the soil-material continuum
Model approach
Concepts integrated in the model
Modeling conditions
Model results
Limitations of the model
Perspectives and recommendations
Artificial xylem technology by biomimicry to the soil-plant continuum
Bioarrangements used to prevent soil pollution
Heavy metal transport indicator: phytoextraction or phytostabilization
CONCLUSION
![]() Télécharger le rapport complet
Télécharger le rapport complet