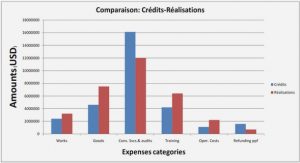
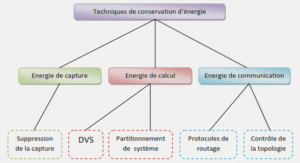
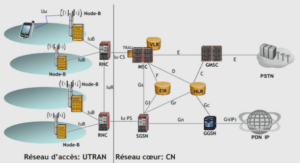
Zeolite Structure
Zeolites are basically made of TO4 tetrahedra, i.e., the primary building units (PBUs) in which the T atom, including Si, Al or some other elements like P, V, Ti, Ga, Ge, B, is covalently bonded to four oxygen atoms, e.g., [SiO4] and [AlO4] tetrahedra. These TO4 building units are then linked together via covalent bonding to form the secondary building units (SBUs) and composite building units (CBUs). In contrast to CBUs, the SBU units which contain up to 16 T atoms, were found by assuming that the entire framework is made of one type of SBU only in a repetitive manner . There are presently 23 observed SBUs as reported in . It is also possible to imagine that a single zeolitic framework is constructed from the attachment of various more complex units together, the CBUs, such as cages, chains, cavities and channels . Rings can be considered as the simplest building units (SBU or CBU), constructed from n TO4 units where n is usually equal to 4, 5, 6, 8, 10 and 12, forming transport windows of molecular dimensions with a maximum opening of about 0.7 nm in aluminosilicate zeolite. In zeotypes, however, larger windows can be formed, for instance, an extra-large window of thirty TO4 tetrahedra (30-ring windows) has recently been reported for germanosilicate zeolite (ITQ-371) .
Zeolite Classification
Characteristic crystallographic data of zeolitic materials were initially used to identify the distinct types of the frameworks which were later documented by the structure commission of the International Zeolite Association (IZA) and published in “Atlas of Zeolite Framework Types” database or on the regularly-updated IZA website . A three letter Framework Type Code (FTC) is assigned by IZA to each unique identified zeolite topology, which has currently reached to over 230 framework types of zeolites and zeotypes. It should be noted that FTC just refers to the framework type, i.e., different zeolitic materials with various compositions can be called by the same code if they share a framework type.
The orderly perforated structure of the zeolites can also be categorized using other properties such as dimensionality of the micropore network, the size of the micropore opening (the window) and chemical compositions, as briefly discussed below.
Depending on micropore interconnectivity and dimensions, zeolites can be divided into three groups including one-, two-, or three-dimensional (1D, 2D and 3D). The micropore opening (size and topology) is directly related to the number of TO4 units on the ring which forms the window. In this regard, aluminosilicate zeolites have been classified into small pore zeolites with eight membered oxygen ring pore window (8-ring), medium pore zeolites with ten-membered oxygen ring pore window (10-ring) and large pore aluminosilicates with twelve-membered-ring pore window (12 ring).
Zeolitic Materials with Short Micropores
Narrowing down the microporous channel length is achievable through crystalline size reduction to nanosize, creating a secondary pore system (meso-macroporosity) within microporous zeolites and designing novel zeolitic architectures such as core@shell, yolkshell and hollow zeolites. These materials offer all desirable features of classical zeolites along with some new aspects emerging from the physical changes. These techniques are providing opportunities to design a catalyst and adsorbent rationally rather than by the traditional trial and error method.
As stated in many review articles, the first two techniques have been recognized as the main viable methods to improve diffusional transport within zeolites . Another direct consequence of such a change would be the increase of the external surface area, i.e.,meso-/macropore surface area. The active sites residing in these places facilitate the conversion of bulkier molecules (> 1 nm), while a considerable amount of remaining micropores, which are now shorter, can (simultaneously) participate in faster conversion of smaller reactant/product molecules with less diffusion limitation, creating a perfect hybrid catalyst. The importance of the external surface area in some catalytic reactions which occur on or near the external surface was demonstrated by providing two practical examples in the recent review paper .Moreover, the large external surface area can perfectly host new organic functional groups which are basically too large to be grafted on the internal micropore surfaces.
Zeolitic Core@Shell with Zeolite as Shell (Core@Zeolite)
Among all types of possible building materials for core@shell composites, crystalline zeolites seem ideal for forming the shell of a core@shell sphere owing to their high thermal/hydrothermal stability, excellent resistance under corrosive conditions, highly ordered pore structure, large specific surface area and micropore volume, shape selectivity, encapsulation properties and intrinsic chemical activity. This class of material includes allzeolite core@shell composites (zeolite@zeolite) as well as core@shell composite with zeolitic shell and non-zeolitic cores. Yolk-shell and hollow zeolitic materials as their close relatives mainly form by applying some changes on the core part of a pre-synthesized zeolitic core@shell composite.
It should be mentioned that zeolitic core@shell materials may or may not be regarded as an alternative for hierarchical meso-macroporous zeolites. According to the definition presented earlier for hierarchical materials, such composites do not provide expected mass transport pattern as regular meso-macroporous zeolitic materials do. In spite of this discrepancy, some reviewers have considered this class of zeolitic core@shells as hierarchical zeolites .The recent review by Schwieger et al. has assigned the hierarchy within these examples as compositional, but not transport-related, i.e., hierarchical porosity. Although having a mesoporous zeolitic shell can establish a real hierarchy system in these materials, it should be remembered that one of the main reasons for using core@shell material is their shape-selective property which is guaranteed by having an entirely microporous zeolitic shell.
Synthesis of Zeolitic Core@Shell
The idea of having a zeolitic layer on a large-size support has inspired material scientists to synthesize zeolitic core@shell reactors which combine numerous desirable properties in a single unit . Zeolitic core@shell materials are composites formed from crystalline zeolite layer coated on a support in a way that the zeolitic shell totally covers the outer surface of the support. Over the past 15 years, a variety of core materials, porous or non-porous, such as polymers, amorphous silica, and metals, alloys and metal oxides, and even different types of zeolites have been used to synthesize either core@shell or hollow materials with a zeolitic shell. Typically, zeolitic core@shell materials are synthesized using one of three different techniques, i.e., seeded growth technique, in-situ method and physical coating.
|
Table des matières
CHAPTER 1 – STATE-OF-THE-ART
1.1 Introduction
1.2 Zeolites as Ordered Microporous Materials
1.2.1 Zeolite History
1.2.2 Zeolite Structure
1.2.3 Zeolite Classification
1.2.4 Zeolite Properties
1.2.5 Zeolite Synthesis
1.2.5.1 Formation Mechanism and Key Synthesis Elements
1.2.6 The Drawbacks of Classical Zeolites- Problem Description
1.3 Proposed Solutions
1.3.1 Large Pore Materials – Pros and Cons
1.3.2 Zeolitic Materials with Short Micropores
1.3.2.1 Nanozeolites- Pros and Cons
1.3.2.2 Hierarchical Zeolite – Pros and Cons
1.3.2.2.1 Hierarchical Zeolitic Structures – Synthesis Approaches
1.3.2.2.2 Macroscopic Structured Hierarchical Zeolites
1.3.2.3 Why Core@Shell and Hollow Zeolites?
1.4 Zeolitic Core@Shell Composites
1.4.1 Definition
1.4.2 History
1.4.3 Zeolitic Core@Shell with Zeolite as Shell (Core@Zeolite)
1.5 Synthesis of Core@Zeolite Materials
1.5.1 Zeolitic Coatings on Macroscopic Structures
1.5.1.1 Synthesis of Macroscopic Coated Zeolitic Structures
1.5.2 Synthesis of Zeolitic Core@Shell
1.5.2.1 Seeded Growth Technique
1.5.2.1.1 History
1.5.2.1.2 Notes on Synthesis Steps
1.5.2.1.3 Pros and Cons
1.5.2.2 In-Situ Technique
1.5.2.3 Physical Coating Technique
1.6. Synthesis of Hollow and Yolk-Shell Zeolitic Material
1.6.1 Templating Technique – Seeded Growth Technique
1.6.2 Post Treatment
1.6.2.1 Dissolution (Selective Desilication)
1.6.2.2 Dissolution-Recrystallization Technique
1.7 Applications
1.7.1 Adsorptive Separation
1.7.2 Catalysis
1.7.2.1 Shape Selectivity
1.7.2.2 Bi-functionality
1.7.2.3 Protecting Layer
1.7.2.4 Sintering Resistance
1.7.2.5 Passivation of External Surface Area
1.7.2.6 Easy Recovery
1.8 Organization of the Thesis
1.9 References
CHAPTER 2 – SYNERGY BETWEEN STRUCTURE DIRECTION AND ALKALINITY TOWARD FAST CRYSTALLIZATION, CONTROLLED MORPHOLOGY AND HIGH PHASE PURITY OF ZSM-12 ZEOLITE
Résumé
Abstract
2.1 Introduction
2.2 Experimental
2.2.1 Chemicals
2.2.2 Zeolite Preparation
2.2.2.1 Benchmark Syntheses
2.2.2.2 Proposed Synthesis
2.2.2.2.1 TEA+ as Organic Template
2.2.2.2.2 MTEA+ as Organic Template
2.2.3 Characterization
2.3 Result and Discussion
2.3.1 TEA+ as Organic Template
2.3.1.1 Issues with the Use of TEA+- Why MTEA+?
2.3.2 MTEA+ as the Organic Template
2.3.2.1 Using MTEAOH as Organic Template
2.3.2.1.1 Crystallization Time- Rapid Crystallization
2.3.2.1.2 Controlled Size and Morphology
2.3.2.2 Effect of Al Source
2.3.2.3 Effect of Water Content
2.3.2.4 Effect of Si Source
2.3.2.5 Effect of Al Content
2.3.2.5.1 Al-Rich ZSM-12
2.3.2.5.2 Pure Silica ZSM-12
2.4 Conclusions
2.5 Acknowledgement
2.6 Supporting Information
2.6.1 Experimental Section
2.6.1.1 MTEA+-Based ZSM-12 Benchmark
2.6.1.2 TEA+-Based ZSM-12 Benchmark
2.7 References
CHAPTER 3 – SYNTHESIS OF MICROPOROUS/MESOPOROUS CORE-SHELL MATERIALS WITH CRYSTALLINE ZEOLITIC SHELL AND SUPPORTED METAL OXIDE SILICA CORE
Résumé
Abstract
3.1 Introduction
3.2 Experimental
3.2.1 Pure Mesoporous Silica@Silicalite-1 Preparation
3.2.2 Preparation of Metal-Containing Mesoporous Silica Microspheres
3.2.3 Preparation of Supported Metal Oxide Mesoporous Silica@Silicalite-1
3.2.4 Materials Characterization
3.3 Results and Discussion
3.3.1 Effect of Secondary Growth Repetitions Using Pure Silica Cores
3.3.2 Effect of Surface Modifications Using Metal-Containing Cores
3.4 Conclusion
3.5 Acknowledgements
3.6 Supporting Information
3.7 References
CHAPTER 4 – ZEOLITIC CORE@SHELL ADSORBENTS FOR THE SELECTIVE REMOVAL OF FREE GLYCEROL FROM CRUDE BIODIESEL
Résumé
Abstract
4.1 Introduction
4.2 Experimental Section
4.2.1 Preparation of Biodiesel
4.2.2 Synthesis of the Core@Shell Adsorbents
4.2.2.1 Preparation of Silicalite-1 Nanocrystals
4.2.2.2 Preparation of Core Materials
4.2.2.3 Preparation of Core@Shell Products
4.2.3 Material Characterization
4.2.4 Glycerol Adsorption Test
4.2.5 Analytical Methods
4.3 Results and Discussion
4.3.1 Synthesis and Characterization of the Sorbents
4.3.2 Purification of Crude Biodiesel
4.3.2.1 Glycerol Adsorption from Methanol-Free Biodiesel
4.3.2.2 Glycerol Adsorption from Methanol-Free Biodiesel at Elevated Temperatures
4.3.2.3 Glycerol Adsorption from Methanol-Containing Biodiesel
4.3.2.4 Glycerol Adsorption from Methanol-Containing Biodiesel at Elevated Temperature
4.4 Conclusions
4.5 Acknowledgements
4.6 Supporting Information
4.7 References
CHAPTER 5 – GENERAL CONCLUSIONS AND PERSPECTIVES
5.1 Future Work
![]() Télécharger le rapport complet
Télécharger le rapport complet