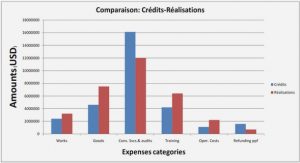
Temporal change in the taxonomie composition of large cells
From September to November 2003, 73 taxa and 46 species of protists were recorded in newly formed sea ice and surface waters of the Canadian Beaufort Sea. Similar numbers (81 taxa and 71 species) were reported during the faU freeze-up in the Laptev Sea (Tuschling et al. 2000). In newly forrned sea ice, we observed two times more protist taxa and species than in the Laptev Sea (32 taxa and 25 species: Tuschling et al. 2000) and Stefansson Sound in November (24 taxa and 18 species: Homer & Schrader 1982).
During our study, the assemblage of the newly formed sea ice was composed of flagellates, diatoms and dinoflagellates. Similar taxonomie compositions were observed in the Laptev Sea (Tuschling et al. 2000) and Greenland Sea (Gradinger & Ikavalko 1998) in early fall. However, the sea-ice assemblage was numerically dominated by penna te diatoms in Stefansson Sound (Homer & Schrader 1982). In the surface waters, we found that unidentified flagellates were the most common group of protists. Similarly, the surface water assemblage was dominated by unidentified flagellates < 6 µm in Stefansson Sound (Homer & Schrader 1982) and by pico- and nanoflagellates in Greenland Sea (Gradinger & Ikavalko 1998).
In the present study, almost all species observed in surface waters were present in the newly formed sea ice while only a few (e.g., Chaetoceros convolutus f. trisetosa, Chaetoceros sp. 6, Pterosperma marginatum, Meringosphaera mediterranea and eight species of Dinophyceae) were found exclusively in the water column . In the Laptev Sea, in contrast, few algal species (i.e., Attheya septentrionalis, Chaetoceros wighamii, Cylindrotheca closterium, Navicula directa, Nitzschia frigida, thecate dinoflagellates and unidentified flagellates) were found in both habitats and two-thirds of those reported in sea ice (eleven diatom species, four dinoflagellates and one chlorophyte) were not observed in the water column (Tuschling et al. 2000). The shallow waters of the Laptev Sea (ca. 46 m), compared to the greater depths of the Canadian Beaufort Sea (up to 810 m) seem to have favored the entrapment of benthic species in the newly formed sea ice.
Selective incorporation of large cells in sea ice
The average abundance of protists >4 µm was almost three times higher in newly formed sea ice than in the underlying surface waters in the Canadian Beaufort Sea. In addition, photosynthetic eukaryotes showed the same distribution as protists, with a lower relative abundance of small cells (<2 µm) and a higher relative abundance of large cells (>4 µm) in newly formed sea ice than in the underlying surface waters . This indicates a selective incorporation of larger ceUs, mainly pennate diatoms , in newly formed sea ice. The selective incorporation of large protists in sea ice has also been demonstrated using an enrichment index for diatoms and autotrophic and heterotrophic flagellated cells > 10 flm in newly formed sea ice off Greenland (Gradinger & Ikavalko 1998). Using the same index as Gradinger & Ikavalko (1998), Riedel et al. (2007b) showed that the newly formed sea ice of the Canadian Beaufort Sea was significantly enriched in large photosynthetic cells (> 5 µm) in the fall. They proposed that the clear selection for the large photosynthetic cells is likely due to cell size and the presence of exopolymeric substances, which greatly enhance the stickiness of cell surfaces.
Sorne species that were incorporated in the newly formed sea ice in the faU of 2003 were present in the bottom landfast ice in Franklin Bay in the late winter and spring of 2004. These species were mainly pennate diatoms (Cylindrotheca closterium, Entomoneis spp., Fragilariopsis cylindrus, Navicula directa, Nitzschia frigida, N. longissima and Pseudo-nitzschia cf. pseudodelicatissima), Dinophyceae (Amphidinium cf. sphenoides, Dinophysis cf. acuminata, Heterocapsa arctica) and Cryptophyceae (Plagioselmis prolonga) (Rozanska, unpublished data). These species are commonly observed at the bottom surface of the sea ice in many regions of the Arctic during the spring and summer (Hsiao 1980, Homer & Schrader 1982, Poulin 1990a, Okolodkow 1992, 1993, Booth & Homer 1997).
Ecological importance of small cells
Recent studies conducted in the Arctic Ocean and adjacent seas have shown that algal abundance, biomass and production in sea ice and surface waters can be dominated by pico- (0.2-2 µm) and nanoalgal (2-20 µm) cells at different periods of the year (e.g., Gosselin et al. 1997, Lovejoy et al. 2002, 2006, Sherr et al. 2003). These small cens are known to be an active component of the microbial food web within the sea ice (Riedel et al. 2007a, 2008) and in the upper water column (Sherr et al. 2003), despite low ambient tempe ratures .
Small-sized algae( 4 µm) were the most abundant cens in sea ice and the underlying surface waters of the Canadian Beaufort Sea in the faIl; however, they were less numerous in sea ice (418-3051 x 103 cells L- 1) than in surface waters (1393-5373 x 103 cens L-1). Not et al. (2005) reported photosynthetic picoeukaryote abundances almost twice as high in the Barents Sea in late summer (2600-10,200 x 103 cells L-1) . However, we were unable to identify the sman photosynthetic eukaryotes. Pigment analyses on samples coIlected in the same area revealed the recurrent predominance of eukaryotic picoalgae from the Prasinophyceae, a class of green algae, in the surface waters throughout the year (Lovejoy et al. 2007). Since the most abundant autotrophic cens were Micromonas-like picoprasinophytes, it is possible that this taxon also dominated in our samples.
Flow cytometry analyses allowed us to distinguish between photosynthetic eukaryotes and prokaryotes (cyanobacteria) based on the presence of the phycoerythrin pigment. In this study, aIl enumerated algae were eukaryotes except at the brackish water (salinity of 16.2) station 1 located in the Mackenzie River plume . At this station, photosynthetic prokaryotic cells 2 µm made up 0.6% and 0.8% of aIl cells < 20 µm in new ice and underlying surface waters, respectively. Their average abundance was three times higher in the underlying surface waters (250 x 103 cells L- 1) than in sea ice (85 x 103 cells L- 1) .
Survival strategies of protists in sea ice
Newly formed sea ice provides a unique habitat for planktonic organisms, albeit one exerting drastic abiotic changes (Gleitz & Thomas 1992). At the end of the summer growth season, sorne phytoplankton species can survive entrapment in newly formed sea ice by continuing to be metabolically active (Gleitz & Thomas 1992, Gradinger & Ikavalko 1998), while others may form resting spores or cysts, using the ice as an overwintering platform (Garris on & Buck 1985). Cyst formation is well-known in Antarctic regions (Garrison & Buck 1989, Buck et al. 1992, Stoecker et al. 1992, 1997, Montresor et al. 1999), but records from the Arctic are very scarce (Ikavalko & Gradinger 1997, Okolodkov 1998).
In the fall, the protist assemblages trapped in newly formed sea ice were still active, as shown by their active uptake of dissolved silicon and nitrate and production of ammonium (Riedel et al. 2007a). In addition to living cells, the newly formed sea-ice assemblage was composed of diatom resting spores and dinoflagellate cysts. These accounted for only a very small proportion (1.8%) of the total protist assemblages, and the majority belonged to different Chaetoceros species. Similar results were obtained by Zhang et al. (2003) from dark survival experiments conducted over a five-month period on ice algae from the autumnal community off Greenland. These authors observed spore/cyst formation in less than 4.5% of ail ceIls, and only for Chaetoceros spp. and dinoflagellates. We can conc1ude that the formation of spores and cysts is a minor survival strategy for Arctic sea-ice protists.
Snow cover effect on net observed growth rate, ceIl abundance and taxonomic
composition of ice protists
Throughout the sampling period, the net observed growth rate, abundance and taxonomie composition of the bottom ice photosynthetic protists were influenced by snow cover depth, which strongly influences light transmission through the ice sheet (Maykut 1985, Perovich 1990, Belzile et al. 2000). Indeed, diatom abundance was significantly lower under high snow cover, while nanoflagellates and dinoflagellates showed no differences between the two snow depths .In addition, the bottom ice algal bloom, which was mostly composed of pennate diatoms, was delayed by one week under high snow cover, as mentioned previously. These results indicate that diatoms
were more affected by light conditions than nanoflagellates and dinoflagellates. By the end of February, the bottom ice irradiance was sufficient to allow diatom growth under low snow cover. Unfortunately, sub-ice irradiance was measured only on one occasion prior to the bloom period. On 18 March, the sub-ice irradiance was 2.6 and 5.8 µmol photons m-2 S-I under high and low snow cover, respectively. These values are within the range of irradiance sufficient to trigger the growth of autotrophic protists in the bottom ice horizon (i.e., 2-9µmol photons m-2 S- I: Homer & Schrader 1982, Gosselin et al. 1985).
Under low snow cover sites, the net observed growth rates of diatoms and nanoflagellates were significantly higher before (0.15-0.23 d-I) than during (0.03-0.09 d-I) the bloom period . This seasonal decrease in net observed growth rates was also observed for algae determined by epifluorescence microscopy from the same sampling site (Riedel et al. 2008). This general pattern of decreasing net observed growth rate, as the biomass of protists accumulates in the environment, is similar to that observed during phytoplankton development in a stratified water column (Parsons et al. 1984b). The smaller net observed growth rates later in the season may result from losses of bottom ice protist cells by sinking, grazing, virallysis and/or ablation.
|
Table des matières
INTRODUCTION GÉNÉRALE
The Changing Aretie Environment
Importance of Sea lee
Eeological Role and Importance of lee Aigae in Polar Eeosystems
Sea-lee B iota
Annual Cycle of the Protist Community in Sea lee
Taxonomie Composition
Role of Environmental Factors
lrradiance, lee Thiekness and Snow Cover
Nutrients
Salinity
General Objectives
CHAPITRE 1 : PROTIST ENTRAPMENT IN NEWLY FORMED SEA ICE IN THE COASTALARCTIC OCEAN
RÉSUMLÉ
ABSTRACT
1.1. Introduction
1.2. Materials and methods
1.2.1. Study site and sampling
1.2.2. Laboratory analyses
1.2.3. Statistical analyses
1.3. Results
1.4. Discussion
1.4.1. Temporal change in the taxonomie composition of large cells
1.4.2. Selective incorporation of large cells in sea ice
1.4.3. Ecological importance of small cells
1.4.4. Survival strategies of protists in sea ice
1.5. Conclusion
CHAPITRE II :INFLUENCE OF ENVIRONMENTAL FACTORS ON THE DEVELOPMENT OF BOTTOM ICE PROTIST COMMUNITIES DURING THE WINTER-SPRING TRANSITION
RÉSUME
ABSTRACT
2.1. Introduction
2.2. Materials and methods
2.2.1. Sampling and laboratory analyses
2.2.2. Statistical analyses
2.3. Results
2.4. Discussion
2.4.1. Seasonal and short-term variability
2.4.2. Snow cover effect on net observed growth rate, cell abundance and taxonomie
composition of ice protists
2.4.3. Heterotrophic organisms
2.4.4. Key species
2.4.5. Influence of nutrient supply on the large-scale horizontal distribution of bottom
ice algae
2.5. Conclusion
CHAPITRE III :SMALL-SCALE HORIZONTAL DISTRIBUTION OF BOTTOM ICE PROTISTS
DURING THE VERNAL SEASON IN THE WESTERN CANADIAN ARCTIC
RÉSUME
ABSTRACT
3.1. Introduction
3.2. Materials and methods
3.2.1. Study site and sampling
3.2.2. Laboratory analyses
3.2.3. Statistical analyses
3.3. Results
3.3.1. Temporal variability
3.3.2. Horizontal variability
3.3.3. Distribution of bottom ice protists and spatial processes
3.3.4. Environnmental variables and taxonomie composition
3.4. Discussion
3.4.1. Seasonal variation
3.4.2. Horizontal variation
3.5. Conclusion
CONCLUSION GÉNÉRALE
RÉFÉRENCES
![]() Télécharger le rapport complet
Télécharger le rapport complet