It is now well accepted that a low consumption of fatty foods, regular physical activity and a high consumption of plant-derived foods help maintain a good health status. In particular, there is an association between an increased level of fruits and vegetables in the diet and a reduced risk of some life-threatening diseases such as cardiovascular diseases and cancer (Parr & Bolwell, 2000). There is growing acceptance that many phenolic secondary metabolites (polyphenols) present in foodstuffs may exert beneficial effects in the prevention of these degenerative diseases (Del Rio et al., 2010). Over the last few decades, the worldwide consumption of citrus fruits and juices has been increasing, thereby stimulating the research on the most abundant bioactive citrus phenols, i.e. FLAVANONES.
Chemistry and Classification:
Polyphenols are classified into two major classes: Flavonoids and NonFlavonoids. The later one includes the structurally simple molecules such as phenolic acids (hydroxybenzoic acids and hydroxycinnamic acids) and stilbenes, and complex molecules comprising of stilbene oligomers, tannins and lignins (Cheynier, 2005). The former, the most studied subclass of polyphenols, represents about more than 9000 identified compounds (Marten and Mithöfer, 2005; Pietta, 2000). Flavonoids commonly share the same generic structure, the flavan nucleus, consisting of two aromatic rings (A and B) linked by an oxygen-containing pyran ring (C). Differences in the linkage of aromatic ring (B) to the benzopyran (chroman) moiety (A and C) allow to distinguish between flavonoids (2-phenylbenzopyrans), isoflavonoids (3 benzopyrans), and neoflavonoids (4-benzopyrans) (Fig. 01). The 2- phenylbenzopyrans are further divided into two groups depending on the presence of a hydroxyl group at position C-3 of C-ring. These include: 3-hydroxyflavonoids, which contain a hydroxyl group (flavonols, flavanols, anthocyanidins, dihydroflavonols), and 3- deoxyflavonoids, which are short of a hydroxyl group (flavanones and flavones). Flavones differ from flavanones by a C2-C3 double bond (Fig. 02) (Marais et al., 2006). The flavanone class encompasses an array of compounds with simple and complex structures referring to their O- and/or C-substitutions (hydroxy, methoxy, methylenedioxy, C-methyl, Chydroxymethyl, C-formyl groups), isoprenoid substituents (noncyclic isoprenoid group, furano or dihydrofurano rings, dimethylpyrano or dimethyldihydropyrano rings), C-benzyl groups, stilbene and anastatin moieties, conjugations to phenolic acids, and diarylheptanoid attachments (Veitch and Grayer, 2006).
Biosynthesis of Flavanones in Plants:
Due to the diverse physiological functions in plants and beneficial nutritional effects, flavonoids are now attractive targets for genetic engineering strategies with aim to produce plants having high nutritional value by modifying the flavonoids biosynthesis. In most of the plant species, the flavonoid biosynthetic pathway has been almost completely elucidated. In general, the biosynthesis of flavonoids is initiated by two precursors named Malonyl-CoA and p-Coumaroyl-CoA which are originated from carbohydrate metabolism and phenylpropanoids pathway, respectively. After the condensation of three molecules of malonyl-CoA with one molecule of p-coumaroyl CoA, yellow coloured chalcones are formed which consist of two phenolic groups attached by an open three carbon bridge. This enzymatic initiated step is catalysed by chalcone synthase. The unstable chalcone form is normally isomerised by the enzyme chalcone isomerase to form the corresponding flavanone. Flavanones are the backbone of this biosynthesis pathway as based on them all other flavonoid classes are generated like flavones, isoflavones, flavanols, flavonols and anthocyanidins (Fig 03) (Schijlen et al., 2004; Marten and Mithöfer, 2005). Moreover, in citrus species, UDP-glucose flavanone-7-O-glucosyltransferase (UFGT) and UDP rhamnose flavanone glucoside rhamnosyltransferase (UFGRT) sequentially convert the flavanone aglycones into their glucosides and rhamnoglucosides (Lewinsohn et al., 1989).
This biosynthetic pathway is highly exploited by agronomists, plant pathologists, soil scientists, and biologists to study the role of phenolic compounds in different plant physiological functions such as insect-plant interaction (Simmonds, 2001), pigmentation (Mato et al., 2000), heavy metal tolerance (Keilig and Ludwig-Müller, 2009), disease resistance and UV-scavenging (Cooper-Driver and Bhattacharya, 1998). Recently, Fowler and Koffas (2009) have reviewed the biotechnological production of flavanones by using various microorganisms. On the other hand, some works deal with trying to produce lower levels of flavanones in plants. For example, an Agrobacterium-mediated genetic transformation approach has been used to reduce the naringin contents (due to its bitter taste) in Citrus paradisi Macf. (grapefruit). A decrease in leaf naringin levels was obtained by targeting the chalcone synthase (CHS) and chalcone isomerase (CHI) genes (Koca et al., 2009).
Diversity and Distribution of Flavanones:
Fruits and vegetables are rich sources of micronutrients such as vitamins and antioxidants. Among these phytochemicals, flavanones are widely distributed in about 42 higher plant families especially in Compositae, Leguminosae and Rutaceae (Iwashina, 2000). A few decades ago, flavanones were only considered minor flavonoids (see Bohm in the three volumes of “The Flavonoid Advances in Research”, Ed. J. B. Harborne, published between 1975 and 1994), like chalcones, dihydrochalcones, dihydroflavonols and aurones. However, during the last 15 years, the total number of known flavanones has become so large that they now appear among the major flavonoid classes like flavones, isoflavones, flavanols, flavonols and anthocyanidins (see Veitch and Grayer in “Flavonoids – Chemistry, Biochemistry and Applications”, Eds Andersen and Markham, 2006).
Based on the criterion of flavanone content, citrus plants belonging to the Rutaceae family appear especially important. Depending on the plant type, flavanones can be found in all plant parts, above- and below-ground, from vegetative part to generative organs: stem, branches, bark, flowers, leaves, roots, rhizomes, seeds, fruits, peels etc. Beside the aglycone forms, flavanones are also present along with their conjugates. They can be classified into several subgroups depending on their O substitution (OH and OMe), C-methylation, Cprenylation and C/O-glycosylation (Veith & Grayer, 2008). Up to now about 350 flavanone aglycones and 100 flavanone glycosides have been discovered in nature (Iwashina, 2000). The glycosidic forms represent a significant proportion of the conjugated flavanones .
|
Table des matières
RESUME
ABSTRACT
LISTE DES COMMUNICATIONS
LISTE DES TABLEAUX
INTRODUCTION
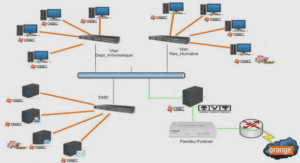
PRESENTATION DE L’UNITE MIXTE DE RECHERCHE « SECURITE ET QUALITE DES PRODUITS D’ORIGINE VEGETALE »
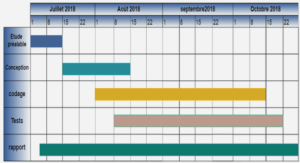
AVANT-PROPOS
BIBLIOGRAPHIE SUR FLAVANONES (Chapitre 1)
1.1. Chimie et Classification
1.2. Biosynthèse
1.3. Diversité et Distribution
1.3.1. Introduction
1.3.2. Naringenine
1.3.3. Hespéretine
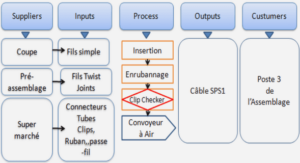
1.4. Extraction
1.5. Synthèse
1.5.1. Aglycones
1.5.2. Chalcones
1.5.3. Glycosides
1.5.4. Glucuronides
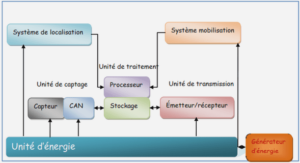
1.6. Biodisponibilité
1.6.1. Introduction
1.6.2. Métabolisme
1.6.3. Pharmacocinétique
1.7. Liaison avec la sérum albumine
1.8. Bioactivité
1.8.1. Introduction
1.8.2. Propriétés antioxydantes
1.8.3. Effets anti-inflammatoires
1.8.4. Effets anti-cancer
1.8.4.1. Effets anti-mutageniques
1.8.4.2. Effets anti-tumoraux
1.8.4.3. Effets anti-prolifération
1.8.5. Effets cardiovasculaires
1.8.5.1. Effets vasorelaxants et vasoprotecteurs
1.8.5.2. Effets sur les maladies coronariennes
1.8.5.3. Effets anti-athérogènes
1.8.6. Autres
REFERENCES BIBLIOGRAPHIQUES
PUBLICATION N° 1 (Chapitre 2)
PUBLICATION N° 2 (Chapitre 3)
PUBLICATION N° 3 (Chapitre 4)
PUBLICATION N° 4 (Chapitre 5)
PUBLICATION comme Co-auteur N° 5 (Chapitre 6)
DISCUSSION GENERALE ET CONCLUSION
![]() Télécharger le rapport complet
Télécharger le rapport complet