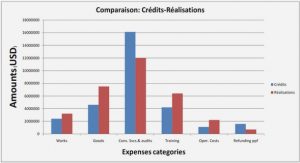
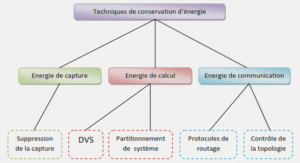
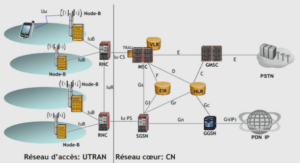
Plasma polymerization
Plasma polymerization involves thin film deposition, a technique for fabricating thin polymer coating from almost any organic or inorganic precursor in order to create hydrophilic/hydrophobic surfaces. Plasma polymerization offers a number of advantages over other polymerization methods. The most significant advantage of plasma polymerization is its ability to produce polymer films of organic compounds that do not polymerize under normal chemical polymerization conditions. Nearly all monomers, even saturated hydrocarbons and organic compounds without a polymerizable structure such as a double bond, can be polymerized with this technique. A second advantage is the ease of application of the polymers as coatings versus conventional coating processes. While coating a substrate with conventional polymers requires a number of steps, plasma polymerization essentially accomplishes all these in a single step. This leads to a cleaner synthesis and coating process, since no solvent is needed during the polymer preparation; no cleaning of the resultant polymer is needed either.
In this process, the growth of low molecular weight molecules (monomers) into high molecular weight molecules (polymers) occurs with the assistance of plasma energy, which involves activated electrons, ions, and radicals. In low-pressure plasma polymerization, generally, the liquid precursor is vaporized in an evaporator pumped into the vacuum chamber. The excited electrons created in the glow discharge ionize the monomer molecules. The monomer molecules break apart (fractionate) to create free electrons, ions, exited molecules, and radicals. The radicals adsorb, condense, and polymerize on the substrate .The electrons and ions crosslink, or create a chemical bond, with already deposited molecules, creating a harder and denser coating.
The important advantages of depositing thin films via low pressure plasma polymerization are :
> The precursor used does not need to contain the type of fiinctional groups normally associated with conventional polymerization.
> Deposition is achieved without the use of solvents (environmentally benign process).
> Such films are often highly coherent and adherent to a variety of substrates, including glass and metal surfaces.
> Less time is needed for the processes involved, such as coating, curing, loading and/or unloading, and part transfer.
> The thickness of plasma polymerization films can be easily varied from lnm to several mm’s.
> Plasma polymerization films can be deposited directly onto an activated substrate without breaking vacuum.
> The deposited film has good corrosion resistance.
> Through careful control of the plasma polymerization parameters, plasma polymerization films can be tailored to contain specific functional groups.
Plasma polymerization is a specific type of plasma chemistry, and many factors can affect the chemistry and morphology properties of the resulting polymer. The most important plasma process parameters are deposition time, input power, monomer flow rate, type of carrier gas, distance from the precursor injection point, and type of both precursor and carrier gas. In the following section, the effect of these plasma process parameters on the surface wettability, surface roughness and/or surface chemical composition is discussed.
Influence of precursor chemistry
The choice of monomer is of primary importance in order to create the superhydrophobic coating. The most popular precursors, which are used for the fabrication of hydrophobic coatings by plasma polymerization, are organosilicon precursors and fluorocarbon precursors.
The plasma polymerized fluorocarbon precursor displays several advantages, including low coefficient of friction, low surface tension, good thermal stability, good biocompatibility, and chemical resistance [12]. A vast number of publications have investigated types of fluorocarbon precursors with different chain lengths, saturated and unsaturated, linear and cyclic structures, all factor that can affect the water repellency of a coating.
Organosilicon monomers have many advantages, such as stability at elevated temperature, availability, liquid state, safe handling, and low cost. Due to this assets, many studies have preferred the plasma polymerization of different organosilicon precursors in order to create a hydrophobic coating.
Influence of input power on wettability
One of the most important plasma operating parameters is input plasma power. This parameter allows control over precursor fragmentation in the plasma and the physicchemical properties of the deposited film, considering that low input power results in decreased fragmentation of precursor as well as an increase in the linear structure of the coating. Nevertheless, for high input power, the high fragmentation of precursor led to an increase in both the cross-linked structure and the roughness of the coating. At the same time, it led to a decrease in desired groups. Many studies showed the effect of plasma polymerization power on the contact angle of the resulting coating.
Hegemann et al. [79] investigated plasma polymerized HMDSO films on polycarbonate (PC) substrates in an effort to generate hydrophobic surfaces. Their results showed that a low plasma power input yielded the low fragmentation and high linearization of the monomer. As a result, and due to an increase of methyl groups’ content, a hydrophobic coating was created. In addition, they showed that the content of methyl groups was reduced through stronger fragmentation of the HMDSO molecules by increasing the power input. The end result was improved mechanical stability.
Influence of deposition time on wettability
Several studies have shown that deposition time is an important parameter in controlling the thickness of the coating as well as the deposition rate. However, increasing the deposition time can increase the presence of hydrophobic functions.
Kale et al. [31] showed that by increasing the deposition time in the same plasma conditions, the chemical nature of the deposited polymer is similar and consequently, the variation of contact angle remains constant.
Influence of monomer flow on wettability
The monomer flow rate is another parameter that can affect the wettability of a coating. By increasing the monomer flow rate, the concentration of methyl groups can also increase. However, it may lead to coating delaminate.
Kale et al. [31] observed the effect of monomer flow on wettability of atmospheric pressure plasma polymerized Hexamethyldisiloxane coating on cotton fabrics. They found that by increasing the monomer concentration, the surface energy changed smoothly.
According to the results of Morent et al. [89], the contact angle of atmospheric pressure plasma polymerized Hexamethyldisiloxane increased from 99° to 108° by accelerating the concentration monomer flow from 1.3 to 3 ppm. They showed that by further increasing the concentration of monomer flow to 4 ppm, the contact angle remained constant.
|
Table des matières
CHAPTER I INTRODUCTION
1-1- Research problem description
1-2- Objective
I-3-Statement of Originality
1-4- Overview of thesis
CHAPTER II REVIEW OF BACKGROUND LITERATURE
Introduction
II-l- Wettability and superhydrophobicity
II-2-Icephobicity
II-3- Plasma treatment
II-4- Plasma polymerization
II-4-1- Influence of precursor chemistry
II-4-2- Influence of input power on wettability
II-4-3- Influence of deposition time on wettability
II-4-4- Influence of monomer flow on wettability
II-4-5- Influence of distance from monomer inlet on wettability
II-4-6- Influence of pre-treatment and different carrier gas
II.5-Stability
II-5-1-UV degradation
II-5-2-Effect of different pH
II-5-3-Corrosion resistance
II-6-Conclusion
CHAPTER III EXPERIMENTAL APPROACH
Introduction
III-1 – Thin film deposition process
III-l-l- Preparation of micro/nanostructure roughness
III-1-1-1-Anodization method
III-1-1-2- Immersion in boiling water
III-1-2-Plasma polymerization process
III-2- Design of Experiment (DOE) technique
III-3- Analytical methods
III-3-1-Wettability
IÏI-3-1-1 -Static contact angle measurement
III-3-1-2- Dynamic contact angle measurement
III-3-1-2-1-Contact angle hysteresis
m-3-l-2-2-Sliding angle (SA)
III-3-2- Surface morphology
III-3-2-1-Atomic force microscopy (AFM)
IÏI-3-2-2- Scanning electron microscopy (SEM)
III-3-3- Surface chemical composition
III-3-3-1- Fourier transform infrared spectroscopy (FT-IR)
III-3-3-2- X-ray photoelectron spectroscopy (XPS)
III-3-4- Coating thickness measurement (Ellipsometry)
III-3-5- Ice adhesion test
III-3-5-1-Icing wind tunnel
III-3-5-2-Centrifugal instrument
III-3-6- QUV accelerated weathering Tester
III-3-7- Electrochemical test (Corrosion)
CHAPTER IV PLASMA POLYMERIZED HMDSO COATING
Introduction
IV-1- Creation of surface roughness
IV-1-1-Anodization process
IV-1-2- Boiling water treatment
ÏV-2- Process of plasma polymerized HMDSO coating
IV-3- Optimization of plasma process parameters
ÏV-4- Conclusion
CHAPTER V STUDY OF THE STABILITY OF THE PLASMA POLYMERIZED HMDSO COATING
Introduction
V-1-Effect of UV radiations on HMDSO thin films
V-l-1- Effect of UV exposure on a HMDSO coating deposited on an anodized aluminum
surface
V-l-2- Effect of UV exposure on the HMDSO coating on a water-treated aluminum
surface
V-2- Effect of different pH solution and immersion in distilled water on wettability of
HMDSO coating
V-2-1- Stability of HMDSO coating on an anodized aluminum surface immersed in
various pH solutions
V-2-2- Stability of a HMDSO coating on a water-treated aluminum surface immersed in
various pH solutions
V-3-Icephobicity
V-3-1- Ice adhesion measurement of a PP-HMDSO coating
V-3-2- Study of the stability of a coating against several icing/de-icing cycles
V-3-2-l-Ice adhesion of a HMDSO coating deposited on an anodized aluminum
surface
V-3-2-2-Ice adhesion of a HMDSO coating deposited on a water-treated aluminum
surface
V-4-Conclusion
CHAPTER VI IMPROVEMENT OF THE STABILITY OF A PLASMA POLYMERIZED HMDSO COATING
Introduction
VI-1- Modification of the plasma parameters
VI-1-1- Influence of deposition time on the thickness of thin films
VI-1-2- Effect of deposition time on the chemical composition of coatings
VI-l-3-Effect of deposition time on the wettability of PP-HMDSO coatings
VI-2- Stability of the coating against UV degradation
VI-2-1-Effect of UV exposure on a PP-HMDSO coating on an anodized aluminum
surface
VI-2-2-Effect of UV degradation of a PP-HMDSO thin film deposited on a water-treated
aluminum surface
VI-3-Effect of various pH solutions and immersion in distilled water on the coating
wettability
VI-3-1- Stability of an HMDSO thin film deposited on an anodized aluminum surface
immersed in different pH solutions
VI-3-2-Stability of a HMDSO thin film deposited on a water-treated aluminum surface
immersed in different pH solutions
VI-4-Ice adhesion strength results
VI-4-1 -Stability of a PP-HMDSO coating against several icing/de-icing cycles
VI-4-l-l-Study of the stability of a PP-HMDSO thin film deposited on an anodized
aluminum surface after several icing/de-icing cycles
VI-4-1-2- Study of the stability of a PP-HMDSO coating deposited on a water-treated
aluminum surface after several icing/de-icing cycles
VI-5- Study of anti-corrosion properties of superhydrophobic surfaces
VI-6-Conclusion
CHAPTER VII CONCLUSION
![]() Télécharger le rapport complet
Télécharger le rapport complet