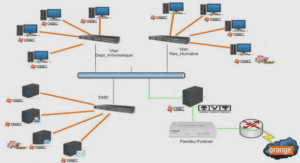
Importance of pH in environmental monitoring
Water is the most common and vital substance on Earth. Pure water is a clear, colorless, and odorless liquid made up of one oxygen and two hydrogen atoms. At 25⁰ C in pure water, the amount of hydrogen and hydroxide ions is equal. That is why pure water is described as a neutral solution. The pH of the water has been identified as one of the most important parameters affecting the quality of the water. In fact, chemical, biological, and physical factors can affect the pH of water (Maciel et al., 2013). Rainwater is naturally acidic, around pH of 5.6. But nowadays, due to the excessive release of CO2 and some pollutants, the rain is becoming excessively acidic (Sumari et al., 2010). CO2 dissolves in water to form carbonic acid as follows: CO2 + H2O H2CO3 (1.2) In some places, the acidity increases to harmful levels between 4.0 and 5.0 pH. Plants will grow better if the soil is maintained at an optimal pH (Warudkar et Dorle, 2016). This variation of the pH of the rainwater is destroying many plantations. For this reason, various environmental agencies are trying to minimize the pollutants that cause acid rain, and pH measurement is a good indicator for environmental monitoring.
Ocean acidification is another alarming environmental issue, essentially caused by the atmospheric carbon dioxide. In fact, like water and air mix at the surface CO2 dissolves in water to form carbonic acid (Irish et al., 2010). Oceans, lakes, and rivers absorb roughly one third of carbon dioxide emissions which represent 22 million tons each day and this gas will increase the acidity of the water. Before the industrialization, the usual pH of the ocean was about 8.2. Current ocean pH is roughly 8.09. This change of 0.11 pH in logarithmic scale may be seen to be harmless but it is equivalent to a 30% increase in acidity, and it is already affecting and stressing the marine life which leads to dying and severely disturbing ecosystems. According to the Australian government, if the emission of CO2 keeps the same tendency, by 2100 it could make the oceans up to 320% more acidic. Then the marine life may not survive in that extreme environment((ASOC), 2010). Many governments are extremely concerned by this problem, Australia is the first concerned because it does not only have an impact on ecology, it also has an economic impact (fisheries, aquaculture, tourism). They are massively investing in remote data monitoring and try to reduce the effect of this phenomenon ((AIMS), 2016). There is a lack of data, which result in a bad model prediction especially data collected in depth water and open ocean ((IMOS), 2016).
Electrodeposition of Iridium oxide
Electrodeposition is the application of a conductive coating to another conducting surface by an electrochemical process. The item to be plated is made the cathode of an electrolysis cell through which an electric current is applied. The article is immersed in an aqueous solution (the bath) containing the desired metal in a soluble form (as cations or as ions). The anode is usually made from noble metal, such as Platinum. IrOx films can be formed by electrodeposition using a potentiostat (We used model 700E, CH Instruments, Austin, TX). Electrodeposition steps are illustrated in Figure 2.8 as follows (Hu et al., 2009): Step 1: We prepared the Yamanaka solution at room temperature as follows. First, 70 mg of IrCl4 (purchased from Artcraft Chemicals, NJ) was dissolved in 50 ml of DI water by stirring for 30 minutes, followed by adding 0.5 ml of H2O2 (30%). After 10 minutes, 250 mg of oxalic acid dehydrates (C2H6O6 from Sigma-Aldrich) was added and the solution was stirred for another 10 minutes. The pH was then adjusted to 10.5 by slowly adding potassium carbonate (K2CO3 from Sigma-Aldrich). The resulting solution was left for four days to stabilize; Step 2: To form the IrOx sensing film, the working electrode was electrodeposited using cyclic voltammetry (CV) feature of the potentiostat with a potential range of -0.8 V to +0.7 V and a scan rate of 0.1 V/s for 50 minutes. Electrodeposition activity which is illustrated in figure 2.8 shows that the current is increasing after each cycle this means that the thickness of electroplated IrOx is increasing too (IrOx has higher electrical resistance than Silver). According to results illustrated in Figure 2.9, we get a linear response, better than the Nernstian response (Called super-Nernstian) with higher sensitivity. In fact, the sensitivity at room temperature (22°C at that time) was equal to 70.66 mV/pH. This non-Nernstian response slopes presented here is explained by the complexation processes in IrOx membrane (Miyake et al., 2012) We also performed a SEM and an EDS to confirm the formation of Iridium oxide; figures 2.10 (a) and (b) show a uniform deposition of IrOx. Figure 2.10 (c) shows a map distribution of each element. As we can see, we have a uniform distribution of Iridium and oxygen.
LTCC based pH sensor
Low temperature co-fired ceramic (LTCC) technology has been used since the 1950s, especially for radio-frequency (RF) and high-frequency applications. Further, it also offers an excellent integration capability (up to 10 layers, feature size can be 15 μm) to reduce the entire device size compared with systems based on printed circuit boards (PCB). For instance, it allows for the production of three-dimensional structures while providing integration with sensors and electronic components in the same ceramic substrate (Pietrikova, 2001). As Au and Ag’s metal traces are used, providing enhancements in conductor thickness compared to sputtering. We used two DuPont’s 951AT layers as a substrate, and all steps followed an industry-standard LTCC fabrication chain (figure 3.11). The first step was to prepare the mask. Next, the traces were printed using a mask and DuPont’s silver paste. A laser-cutting machine was used to create a testing chamber. The two layers were aligned using a high-precision aligner and pressed together using hydraulic pressure. Finally, the batch of sensors was separated using a guillotine and the resulting laminated circuit was co-fired. Further, since the thickness of the printed conductor (shown in figure 3.12, is equal to 9.955 um) is significantly greater than those formed by other methods, such as sputtering (0.9 um); then, LTCC is convenient with both sol-gel and electrodeposition of IrOx. Knowing that electrodeposition allows a better sensitivity; then, we chose this last method.
To form the IrOx sensing film, the WE was electrodeposited using cyclic voltammetry (CV) feature of the potentiostat with a potential range of -0.8 V to +0.7 V and a scan rate of 0.1 V/s for 50 minutes. Figure 3.13 shows the results of a Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS). The results confirm that we get a uniform Iridium oxide formation. To reduce costs and time, as well as to obtain a compact size of the system compared with those using the glass RE electrode, our RE was simply printed using the Ag/AgCl paste. Figure 3.14 LTCC pH sensor calibration at room temperature We used five different solutions with pH values of 4, 5, 7, 8.8 and 10 to calibrate our pH sensor, at room temperature. Buffer solutions with pH of 10, 7 and 4 were purchased from Fisher Scientific, while the ones with pH of 5 and 8.8 were made in house using DI water and buffer solutions. The pH values were determined using a commercial pH meter (SPER Scientific). The calibration results were plotted in figure 3.14, showing a super-Nernstian response with the sensitivity slope of 71 mV/pH. The response was also highly linear (R2 = 0.9988). Microfabrication technique (MEMS) is simply not adapted for the two deposition techniques of IrOx (electrodeposition and sol-gel). In fact, heat treatment generates heat stresses, resulting in the formation of cracks. Also, the use of solvent dissolves the coating during sol-gel. Then, this technique is not a convenient fabrication process for our pH sensor. In terms of simplicity and cost, laser micro-machined pH sensor is a good option; except the fact that, sputtered thin conductor cannot support electrodeposition activity. Then, it is only compatible with sol-gel deposition (Electrodeposited IrOx have a better sensitivity than sol-gel deposited IrOx). LTCC proved to be the most suitable fabrication technique, in fact, it allows a better integration and miniaturization; it is compatible with both electrodeposition and sol-gel deposition of IrOx, and it allows batch production which reduces costs. (Table 3.1 resumes the main advantages and disadvantages of each fabrication technique).
|
Table des matières
INTRODUCTION
CHAPTER 1 FUNDAMENTALS OF pH MEASUREMENT
1.1 pH Definition
1.2 Importance of pH measurement
1.2.1 Importance of pH in environmental monitoring
1.2.2 Importance of pH in food processing
1.2.3 Importance of pH in medical diagnostic and prognostic
1.3 Existing commercial solution for pH measurement
1.3.1 pH Paper strips
1.3.2 Glass electrode
1.3.3 pH Electrodes based on Ion Sensitive Field Effect Transistor
1.4 Conclusion
CHAPTER 2 IRIDIUM OXIDE
2.1 Introduction
2.2 pH Sensing Mechanism of Iridium Oxide
2.3 Different deposition techniques of Iridium oxide
2.3.1 Sol-gel Deposition of Iridium oxide
2.3.2 Electrodeposition of Iridium oxide
2.4 Conclusion
CHAPTER 3 pH SENSOR FABRICATION TECHNIQUES
3.1 Introduction
3.2 MEMS pH sensor
3.2.1 Photolithography
3.2.2 Sputtering
3.2.3 Liftoff
3.2.4 IrOx deposition
3.3 Laser micromachined pH sensor
3.4 LTCC based pH sensor
3.5 Conclusions
CHAPTER 4 NOVEL MINIATURIZED WIRELESS pH SENSING SYSTEMS
4.1 Introduction
4.2 Miniaturized wireless LTCC based pH sensing device
4.2.1 Circuit and layout design
4.2.2 Integration
4.2.3 Wireless user interface
4.3 Other prototypes
4.3.1 Flexible pH sensing device
4.3.2 Glass based microfluidic pH sensing device
4.4 Conclusion
CHAPTER 5 WIRELESS POWER TRANSFER FOR BATTERY-LESS APPLICATIONS
5.1 Introduction
5.2 The concept of wireless power transfer
5.3 Transmitter antenna design
5.3.1 Antenna parameters
5.3.2 Transmitter tuning and performances
5.4 Receiver antenna design
5.4.1 Air core antenna
5.4.2 Flexible spiral antenna
5.4.3 LTCC antenna
5.5 Results and conclusion
CONCLUSION
LIST OF BIBLIOGRAPHICAL REFERENCES
![]() Télécharger le rapport complet
Télécharger le rapport complet