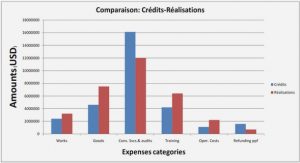
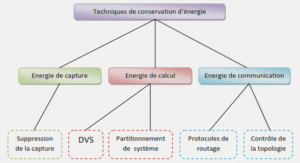
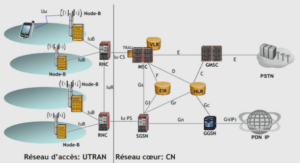
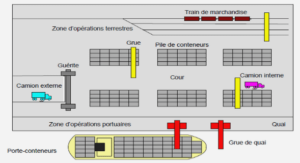
SPECIMENS AND IMAGrNG
An ontogenetic senes of 97 juvenile and adult A. calva specimens was acquired through museum collections and commercial fisheries (Table 1). Since development of the lateral line system is correlated with size rather than age (Münz, ‘ 86; Ledent, 2002), standard length (SL) was used to reconstruct the ontogenetic series. Specimens were photographed in lateral view in order to measure the SL with CT An (Version 1.11.4.2, SkyScan, Bruker-microCT, 2011 , Belgium). SL varied between 15 mm and 545 mm (x = 194 mm; SD = 100 mm). Measurement error was less than one millimetre between two measurements for each specimen. Specimens acquired through fisheries were measured unfrozen, whereas collection specimens were already fixed in fom1alin and preserved in ethanol when measured. SL of one dry skull specimen was estimated using the linear regression between parietal bones (Pa) length and SL for five specimens of similar size (SL = 0.0705*Pa + 5.93; R2 = 0.95). Specimens from fisheries were decapitated, fixed in 4 % neutralized formalin for 24 ho urs and preserved in 70 % ethanol. Bones, sensory canals, and related ramifications were imaged with a micro-CT scan, SkyScan 1173 (Bruker-microCT, 2011 , Belgium). Specimens were preferably scanned while fresh but 42 specimens were scanned after fixation/preservation and three were dry skulls. Fixation/preservation did not affect relative position of components inside the bones (see Appendix 1). Because of important differences in specimen size, the scanning protocol had to be adapted for each skull. For example, 200-mm SL specimens were scanned with an aluminium filter at 100 kV, 80 )..LA, with an exposure of 830 ms and a rotation step of 0.2°. The scanner was set to 55 kV, 100 )..LA, 1400 ms with no filter for smaller specimens « 100 mm) and set to 100 kV, 98 )..LA, 1600 ms with a brass filter for larger specimen (> 300 mm). Resolution was set between 15 and 30 )..Lm. Projection images were reconstructed using NRecon (Version l.6.6.0, SkyScan, Bruker microCT, 2011 , Belgium).
PATTERNING OF RAMIFICATIONS
This study focuses on groups of pores (GP) 16 and 18 of the otic canal, and GP2-3 of the supratemporal commissure (Fig. 1); nomenclature of groups of pores follows Allis (1889). These groups of pores allow testing modularity hypotheses regarding their developmental identity via placodes while taking into account their proximity and their interactions with dermal bones. GPl of the supratemporal commissure was not included in the analyses because it is not comparable to other groups of pores. It has very few ramifications until an advance stage of development, and according to Allis (1889), it develops as a single median group of pores that subdivides to form one group of pores on each extrascapular. Individuals that expressed variation in the presence/absence of certain groups of pores associated with the supratemporal commissure were pooled in order to test for the identity of the missing group of pores. When one of the GP2-3 was absent, the remaining group of pores was identified as 2.5. The distance betweenthe group of pores and the otic canal-supratemporal commissure junction (dis3, dis2 and dis2.5), and the extrascapular width (ew) were measured for both sides of the skull. Measurements were made with CTAn on 2D images digitized with CTVox (Version 2.2.0, SkyScan, BrukermicroCT, 2011 , Belgium). To remove size and magnification effect, statistical analyses were performed on the dis/ew ratio for GP2, GP3 and GP2.5. The effect of allometry was tested by a multiple regression of the dis/ew ratio on size. The null hypothesis of isometry was not rejected by the analysis of variance of the regression (P = 0.6). A Kruskal-Wallis analysis was used to test for differences in relative position of GP2, GP3 and GP2.5. To verify if GP2.5 occupies an intermediate position between GP2 and GP3 , a Student {-test was used to compare its position with the mean position of GP2 and GP3. In order to test if groups of pores tended to be absent on both sides equaUy, the G-test was used, with expected frequencies of 0.5 for both sides. In order to describe the development of ramifications, the groups of pores were schematized. Specimens > 400 mm were viewed with Seg3D (SCI Institute, 2007), a freeware that pro vides tools for volume segmentation. Ramifications were segmented by a paintbrush tool and the isosurface was computed to describe their pattern. Ramifications were fewer in smaller specimens and were thus visualized in sections with CT An and in volume rendering with CTVox. Because size classes were not equally represented, two separate analyses were conducted. Patteming was described for specimens :s 411 mm, whereas the spatial layout was described for specimens 2: 411 mm. Moreover, because ramifications are visualised by X-rays, terminal branches in skin could not be visualized. F or specimens :s 411 mm, each bifurcation was numbered clockwise starting at the root and progressing toward terminal orders (Fig. 2). Ramifications are branching structures that can be treated as trees. An order is the number of branches that separates a given branch from the root. A ramification of a given order that bifurcated to the left was numbered 2n-l, and 2n if it bifurcated to the the right, where n is the number of the ramification of the previous order. The right side of each specimen was inverted so that numbers were equivalent on both sides. Each numbered bifurcation was then coded 1 if present and coded 0 if absent. Bifurcation was taken into account from the moment that there was a constriction between two pores in formation. Fourteen specimens were described twice to compute the error. Schematization error was less than 5 % and it was lower in specimens with fewer ramifications. Development was described with logistic regressions to estimate the SL at which 50 % of the specimens (SLso) have the bifurcation of interest (Fischer-Rousseau et al., 2009; Grünbaum et al., 2012). Significance of logistic regression was tested using the G2 statistic (Quinn and Keough, 2002). The Bonferroni correction was applied to take into account multiple comparisons of regression (significance level adjusted to 0.001).Spearman rank correlation coefficients (rs) were computed to test for shared patterning between sides and between groups of pores. Results from the right and left sides were pooled once symmetry of patterns has been verified.
GEOMETRIC MORPHOMETRICS
As a result of incomplete ossification of the bones in younger specimens, absence or duplication of groups of pores, and in order to limit the analysis to one population (Lac StPierre), only 25 specimens out of 97 were used to verify a priori hypotheses of modularity. Since most specimens were immature, differences in integration between sexes was not taken into account. Raw data obtained with the micro-CT scan were resized and converted to DICOM. 3D landmarks were digitized with Morphome3cs (Version 2.0, Charles University, Prague). Skull landmarks were digitized on the surface and canal landmarks were digitized on sections of DICOM images (Fig. 1 and Table 2). Landmarks were digitized twice on different days by the same user (C.L.). Preliminary analyses and modularity tests were conducted with MorphoJ (Klingenberg, 2011). Each landmark configuration was Procrustes transformed with a join Procrustes fit. Discriminant function analysis (DF A) was used to test for differences in configuration of landmarks between days. Directional and fluctuating asymmetry were assessed with Procrustes ANOVA (Klingenberg and Mclntyre, ’98). Following assessment of measurement enor, observations were averaged and covariance matrices were computed for the symmetric and asymmetric components of the complete dataset. Regression of shape on centroid size was tested for allometry (Monteiro, ’99). Covariance matrices were computed from regression residuals when allometry was significant. We tested three a priori hypotheses of modu1arity regarding the interaction between canals and bones and the interaction among canals (Fig. 3): the dermopterotic and extrascapular and their respective canals are two separate modules (hypothesis 1), the bones and canals are two separate modules (hypothesis 2), the otic canal and the supratemporal commissure are two separate modules (hypothesis 3). A priori hypotheses were tested using Escouffier’s RV coefficient (Escoufier, ’73) as proposed by Klingenberg (2009). Delawney triangulation was used to restrict modules to spatially contiguous landmarks (Klingenberg, 2009). Landmarks were subdivided in subsets of similar size according to hypotheses, and the RV coefficient was computed between subsets. The observed RV coefficient was then compared to a distribution of all other possible RV coefficients when subsets are permuted inside the configuration of landmarks. If the observed RV coefficient is at the lower end (5 %) of the distribution, the partition of landmarks conesponds to the true module boundaries. This method of modularity analysis (MOD) is based on the premise that covariance between modules is low and that covariance is high among elements belonging to a module (Klingenberg, 2009). Two blocks Partial Least Square (PLS) analysis (Rohlf and Corti, 2000) was used to test hypotheses of (1) the dermopterotic and extrascapular and their respective canals as two separate modules and (2) bones and canals as two separate modules (Fig. 2). The RV coefficient measured the conelation between the two sets of landmarks. A permutation test (10,000 rounds) was used to test the hypothesis of complete independence between the two landmark partitions. Although the hypotheses are the same in both approaches (MOD and PLS), PLS analysis differs from MOD because it uses a separate Procrustes fit for each subset. The separate Procrustes fit method is considered more conservative than the join Procrustes fit used in MOD because it ignores covariation related to differences in size between blocks (Klingenberg, 2009).
GROUPS OF PORES PA TTERNING, Y ARIA TION AND MODULARITY
Sensory canal ramification was overlooked for more than a century. As Allis (1889) observed, ramifications have a constant pattern. In our study, ramification development was similar between the left and right sides for GP 16 and GP 18 suggesting that ramifications follow a pre-established patterning (Table 3). Although the development for the groups of pores in the supratemporal commissure was not correlated, we observed that their development is not random. The absence of a significant correlation may result from the absence of groups of pores in this region. To the best of our knowledge, the functional importance of the ramifications has never been tested. However, Janssen (2004) suggested that ramifications serve as filters for small hydrodynamic disturbances which must affect many branches in order to be recorded by the neuromasts. We suggest that the branches must have a constant configuration in order to obtain a reliable signal. However, the position of ramifications could not be investigated with geometric morphometrics due to the close proximity of putative landmarks and variation in the number of landmarks. Sorne groups of pores were missing in about half the specimens, which is more abundant than what Allis (1889) had observed. The absence of a group of pores did not influence the presence or absence of other groups of pores which meet the quasiindependent cri te ri on for modularity; elements of a module are expected to be coherently modified or lost without affecting other structures (Winther, 2001). Moreover, we demonstrated that the two canal sections join in a position (dis2.5) different from the two original positions (dis2 and dis3). Allis (1889) observed that groups of pores were absent when neuromasts were absent. We suggest that the single group of pores develops from canal sections corresponding to neuromasts different from the generalized condition. Franz- Odendaal and Hall (2006) recognized the neuromast as a morphological module. The neuromasts could thus be the module and its modularity is detectable through the development of groups of pores. The number of groups of pores is more variable in the supratemporal commissure than in the otic canal. Because canals and dermal bones may interact in development, it was expected that the extrascapulars, which are the bones associated with the supratemporal commissure, would also vary in number. Among basal actinopterygians [e.g., Lepisosteiformes (Grande, 2010), Acipenseriformes (Hilton and Bernis, ’99)] the number of extrascapulars is frequently variable among and within species. The extrascapular series is also variable among species in different groups of sarcopterygians [e.g., actinistians (Cloutier, ‘ 91), dipnoans (Cloutier, ’97)]. A great disparity in number ofbones and groups of pores could be the result of developmental instability in this region. Moreover, in crown tetrapods, the extrascapulars are lost simultaneously with the supratemporal commissure (Cloutier and Ahlberg, ‘ 96). The joined phylogenetic variation of lateral line canals and dermal bones brings further evidence for their close developmental interaction.
BONE AND CANAL fNTEGRATION
Modularity between dermal bones and canals was not detected. PLS revealed that canals and bones are not independent. However, the covariation was not strong enough to qualify the pair as a module. The otic canal goes through two bones. The interaction between canals is most likely stronger than it is between bones and canals which obscure the bone-canal module. Although the laterai line system has a close relationship to dermal bones because of their formation around canals, other processes are predominant in determining bone shape and sutures. For exemple, feeding was associated with constraint on the skull and variation in suture pattern in the bichir, Polypterus (Markey et al., 2006). A different suture pattern resulting from environmental interaction wouid shift the position of landmarks and reduce their covariation with canal landmarks. Integration has been shown to change with age (Fischer-Rousseau et al., 2009) and our results may not hold for larval and young juveniles as they wouid not have gone through the same deveIopmentai and environmental interactions. Moreover, modularity is a hierarchical concept and developmental processes may act as a palimpsest which can make it difficult to detect modularity when a priori hypotheses are based on only one developmental process (Hallgrimsson et al., 2009).
|
Table des matières
REMERCIEMENTS
RÉSUMÉ
ABSTRACT
TABLE DES MATIÈRES
LISTE DES TABLEAUX
LISTE DES FIGURES
LISTE DES ABRÉVIATIONS, DES SIGLES ET DES ACRONYMES
INTRODUCTION GÉNÉRALE
CHAPITRE 1 LES BLOCS DE CONSTRUCTION DE LA TÊTE D’UN POISSON: MODULARITÉ DÉVELOPPEMENT ALE ET VARIATIONNELLE DANS UN SYSTÈME COMPLEXE
1.1 RESUME EN FRANÇAIS DE L’ARTICLE
1.2 BUILDING BLOCKS OF A FISH HEAD: DEVELOPMENTAL AND VARIATIONAL MODULARITY IN A COMPLEX SYSTEM
1.3 ABSTRACT
1.4 INTRODUCTION
1.5 MATERIAL AND METHODS
1.5.1 Specimens and imaging
1.5.2 Patterning of ramifications
1.5.3 Geometrie morphometrics
1.6 RESULTS
1.6.1 Patterning of ramifications
1.6.2 Geometrie morphometries
1.7 DISCUSSION
1.7.1 Groups of pores patterning, variation and modularity
1.7.2 Modularity of sensory canals
1.7.3 Bone and canal integration
1.7.4 A hierarehieal model of modularity
1.7.5 Comparisons and future work
1.8 ACKNOWLEDGEMENTS
1.9 TABLES
1.10 FIGURES
1.11 ApPENDIX 1
1.12 ApPENDIX 2
1.13 REFERENCES
CHAPITRE 2 CONCLUSION
LISTE DE RÉFÉRENCES BIBLIOGRAPHIQUES
![]() Télécharger le rapport complet
Télécharger le rapport complet