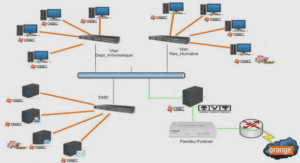
Les dommages indirects à l’ADN
Contexte et objectifs de la recherche
L’ADN est soumis continuellement aux actions des agents environnementaux et des produits métaboliques qui portent atteinte à son intégrité. Ils peuvent induire de l’instabilité génétique, des mutations, et conduire à la mort cellulaire. Parmi eux, les UV sont considérés comme le carcinogène physique le plus important. La moitié des cancers de la peau d’origine kératinocytaire, les carcinomes baso-et spino-cellulaires (CBC, CSC), se développent dans les zones photo-exposées. Selon les statistiques de la Société Canadienne du Cancer l’incidence des cancers de la peau est équivalente à celles de tous les autres cancers réunis et les CBC et les CSC occupant le premier rang (environ 40%) de tous les nouveaux cas de cancer au Canada [363]. Pour empêcher leur développement, deux réponses cellulaires sont cruciales: la réparation de l’ADN endommagé par les UV et la mort des cellules trop endommagées. Une des enzymes activées par les dommages induits à l’ADN par les UV et ayant un rôle dans ces deux réponses est la PARP1. Mes travaux portent sur le rôle de la PARP1 dans la réparation des dommages directs induits à l’ADN par les UV.
Plusieurs études, en commençant avec celles des années 80, ont démontré l’activation de la PARP1 après l’irradiation des cellules aux UVC et UVB. Par la suite, il a été démontré que la PARP1 reconnaît, lie et est activée par les dommages directs, de type T-T, induits par les UV et réparés par la NER [338]. Cependant, les opinions étaient partagées quant au rôle joué par la PARP1 dans la réparation de ces dommages. Quelques études ont rapporté que la PARP1 et son activité catalytique n’avait aucun effet [336, 340, 342] d’autres ont affirmé qu’elle stimulait [364] ou inhibait la réparation des dommages directs induits par les UV [344, 345]. Par conséquent, des études supplémentaires s’avéraient nécessaires afin de prouver sans équivoque la participation de la protéine PARP1 dans la NER des cellules eucaryotes et de déchiffrer le mécanisme exact par lequel cette protéine influence la réparation des dommages directs causés par les UV.
Notre hypothèse est que la protéine PARP1 par son interaction physique avec des protéines de la NER et/ou par sa capacité à modifier ces protéines par son produit, les PAR, est impliquée dans la détection de l’ADN endommagé par les UV et le recrutement de protéines jouant un rôle dans l’étape de reconnaissance des dommages de la NER.
Pour démontrer cette hypothèse, les objectifs spécifiques de mon doctorat sont :
(1) Analyser si l’effet de la PARP1 sur la cinétique de réparation des photo-lésions est universel.
(2) Analyser l’interaction de la PARP1 avec des protéines de la NER et vérifier le rôle
de l’activité de la PARP1 dans ces interactions.
(3) Identifier les protéines acceptrices de PAR parmi les protéines de la NER.
(4) Montrer le recrutement de la PARP1 au niveau du site des dommages induits par les UV in vivo.
Chapitre 3 Role of poly(ADP-ribose) polymerase-1 in the removal of UV-induced DNA lesions by nucleotide excision repair
Mihaela Robu, Rashmi G. Shah, Nancy Petitclerc, Julie Brind’Amour1, Febitha Kandan-Kulangara and Girish M. Shah*
Laboratory for Skin Cancer Research, CHUL (CHUQ) Hospital Research Centre of Laval University, Laval University, Québec (QC) Canada G1V 4G2
1Present address: Terry Fox Laboratory, Vancouver (BC) Canada V5Z 1L3
*Corresponding Author: GM Shah (418) 656 4141/x48259, e-mail:
girish.shah@crchul.ulaval.ca
Avant-propos
Lorsque j’ai amorcé mes études, le rôle de la PARP1 dans la réparation de dommages directs induits à l’ADN par les rayons ultraviolets était un sujet controversé. Les résultats préliminaires obtenus pendant ma maîtrise indiquaient que la PARP1 stimulait la réparation des dommages directs du génome des fibroblastes humains synchronisés en G1. Afin de déchiffrer le mécanisme d’action de la PARP1, nous avons cherchés ses partenaires parmi les protéines de la phase de reconnaissance des dommages de la NER. La caractérisation de l’interaction entre la PARP1 et une de ces protéines, le facteur DDB2, après irradiation aux UVC a fait l’objet d’un article publié en janvier 2013 dans la revue Proceeding of the National Academy of Sciences of the United States of America, lequel est disponible en version PDF à l’annexe 1 de cette thèse.
Les techniques que j’ai mises au point pendant ma maîtrise (l’analyse de la réparation de l’ADN par cytométrie en flux et par immunofluorescence, le fractionnement cellulaire), nous ont permis de démontrer le rôle stimulateur de la PARP1 dans la réparation des dommages directs induits par les UV (Figures 3.1A et C-E). L’effet inhibiteur de la PARP1 sur la réparation des CPD après l’inactivation de son activité catalytique dans la peau des souris avait été observé par Julie Brind’Amour (Figure 3.1B) avant mon arrivée dans le laboratoire du Dr Shah. Je suis également l’auteure des immunoprécipitations montrant l’interaction de la PARP1 avec le facteur DDB2 et la modification de DDB2 avec des PAR in vivo (Figure 3.2C, 3.3A, 3.4C, S3.1D-E), des essais d’immunofluorescence montrant le recrutement de myc-DDB2 (Figure S3.2D) et de la XPC (Figure 3.4E) aux dommages et des fractionnements cellulaires montrant la cinétiques de formation des PAR et le recrutement des protéines de la NER à la chromatine (Figures 3.2A, E, 3.3E, 3.4A, F, S3.3E). Rashmi Shah a réalisé les essais in vitro montrant la stimulation de l’activité de PARP1 par le DDB2 (Figures 3.3C, D, F et S3.3 B-D), la PARylation du DDB2 in vitro (Figures 3.3B et S3.3A) et leur interaction (Figures 3.2B, 3.2D et 3.4B). Nancy Petitclerc a caractérisé l’interaction entre la PARP1 endogène et le DDB2 exogène couplé à une étiquette myc (Figures S3.2 A-C, E). Les clones GMRSiP exprimant une PARP1 exogène couplée à une étiquette FLAG dans des cellules rendues déficientes en PARP1 endogène par interférence à l’ARN, utilisées dans les Figures 3.2B, 3.2D et S3.1B ont été créés par Febitha Kandan-Kulangara. En tant que première auteure, j’ai participé à la rédaction du manuscrit, à sa révision et j’ai fait le montage des figures.
Résumé
Une des réponses précoces des cellules de mammifères aux dommages à l’ADN est l’activation catalytique de l’enzyme nucléaire poly(ADP-ribose) polymérase-1 (PARP-1). La PARP-1 activée forme des polymères d’ADP-ribose (pADPr ou PAR) qui modifient post-traductionnellement des protéines cibles, telles que la PARP-1 elle-même et des protéines de réparation de l’ADN. Bien que ce métabolisme soit connu pour être impliqué dans plusieurs voies de réparation, ici, nous montrons son rôle dans la voie versatile de réparation par excision de nucléotides (NER) qui élimine une grande variété de dommages à l’ADN, y compris ceux induits par les UV. Nous montrons que l’inhibition de la PARP ou la déplétion spécifique de la PARP-1 diminue l’efficacité de réparation des lésions d’ADN induites par les UV du génome de fibroblastes de peau humaine ou de l’épiderme de souris. En utilisant des cellules compétentes et déficientes en NER et des essais in vitro nous montrons que le facteur DDB2, une protéine clé de la phase de reconnaissance des lésions de la sous-voie de réparation globale du génome de la NER (GG-NER), s’associe à la PARP-1 sur la chromatine après irradiation aux UV, stimule son activité catalytique et est modifiée par des pADPr. L’inhibition de la PARP supprime l’interaction du DDB2 avec PARP-1 ou la XPC et diminue la localisation de la XPC à l’ADN endommagé par les UV, qui est l’étape clé menant aux étapes situées en aval de la GG-NER. Ainsi, la PARP-1 collabore avec le DDB2 pour augmenter l’efficacité de l’étape de reconnaissance des lésions de la GG-NER.
Abstract
Among the earliest responses of mammalian cells to DNA damage is catalytic activation of a nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1). Activated PARP-1 forms the polymers of ADP-ribose (pADPr or PAR) that post-translationally modify its target proteins, such as PARP-1 and DNA repair related proteins. While this metabolism is known to be implicated in other repair pathways, here, we show its role in the versatile nucleotide excision repair pathway (NER) that removes a variety of DNA damages including those induced by UV. We show that PARP inhibition or specific depletion of PARP-1 decreases the efficiency of removal of UV-induced DNA damage from human skin fibroblasts or mouse epidermis. Using NER-proficient and deficient cells and in vitro PARP-1 assays, we show that DDB2, a key lesion recognition protein of the global genomic sub-pathway of NER (GG-NER), associates with PARP-1 in the vicinity of UV-damaged chromatin, stimulates its catalytic activity and is modified by pADPr. PARP inhibition abolishes UV-induced interaction of DDB2 with PARP-1 or XPC, and also decreases localization of XPC to UV-damaged DNA, which is a key step that leads to downstream events in GG-NER. Thus, PARP-1 collaborates with DDB2 to increase the efficiency of the lesion recognition step of GG-NER.
Introduction
Mammalian cells respond very rapidly to different types of DNA damage by activation of an abundant and ubiquitous nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1). The activated PARP-1 utilizes NAD+ to form polymers of ADP-ribose (pADPr or PAR) which modify PARP-1 itself and selected target proteins, such as histones and DNA repair proteins [365]. This post-translational modification, i.e., PARylation has been implicated in cellular responses ranging from DNA repair to cell death. Among mammalian DNA repair pathways, PARP-1 has been implicated in the base excision repair, homologous recombination and non-homologous end-joining pathways [304, 366], but we do not know its role in the most versatile nucleotide excision repair (NER) pathway that removes a wide variety of DNA lesions including UV-induced thymine dimers (T-T) and other cyclobutane pyrimidine dimers (CPD) as well as 6-4 photoproducts (6-4PP) [68].
The core mammalian NER pathway uses more than 30 proteins to recognize the damaged site on DNA, remove 24-32 nucleotides long single-stranded DNA containing the lesion, fill the gap using the non-damaged strand as a template and finally ligate the nick [68]. There are two sub-pathways of NER: the transcription-coupled NER (TC-NER) removes lesions from the actively transcribed strands of the genes; and the global genomic NER (GG-NER) repairs lesions from the entire genome. These two pathways differ in the initial step of lesion recognition: TC-NER is initiated when elongating RNA polymerase II stalls at the lesion, whereas GG-NER is initiated when the lesion is recognized in the chromatin context by DDB2 (XPE), which through its participation in UV-DDB-E3 ligase complex ubiquitinates and localizes the key GG-NER protein XPC to the damaged site [146, 186, 367].
Although the roles for different core NER proteins have been well-characterized with bacterial and yeast model systems, we still cannot fully explain the accuracy and rapidity with which mammalian NER is targeted to a very few damaged bases that are surrounded by a large number of unmodified bases in chromatin. In this context, some of the post-translational modifications, such as phosphorylation, acetylation, ubiquitination and sumoylation are known to help different steps of NER [368]. Here, we examined whether PARylation that occurs rapidly after PARP-1 is activated by UV-induced DNA lesions [338], could be involved in improving the efficiency of mammalian NER. Earlier studies examined the effect of impaired PARP-1 function on mammalian NER but obtained contradictory results [345, 369, 370], because the repair of UV-induced CPD was unaffected by PARP inhibition in HeLa cells [369] whereas it was reduced in trans-dominantly PARP-1 inhibited CHO cells [370] or in the PARP-1 impaired triple negative breast cancer cell lines [345]. In view of other confounding factors such as DNA repair defects in CHO or breast cancer cells, we used RNAi or PARP inhibition approaches in multiple mammalian NER proficient models to show that PARP-1 is required for an efficient removal of UV-induced T-T and 6-4PP from genomic DNA. We also show that the catalytic activity of PARP-1 in collaboration with DDB2 leads to an improved function of DDB2 and XPC during the lesion recognition step of mammalian GG-NER.
Results
PARP inhibitors delay removal of UV-induced DNA lesions
To explore the role of catalytic activation of PARP-1 in NER, we first examined the effect of PARP inhibitor PJ-34 on the efficiency of removal of UVC-induced T-T or 6-4PP from genomic DNA of two different human skin fibroblasts using a flow cytometry based assay [371] (Fig. 3.1A). In this assay, the histograms for T-T or 6-4PP at early time points after irradiation (5-15 min) represent initial damage, and movement of histograms at later time points (6-63h) towards untreated cells represents the extent of repair. In the SV-40 immortalized GMU6 human skin fibroblasts, a significant removal of T-T at 24h was seen only in the normal but not in the PJ-34 treated cells (Fig. 3.1A, left panel). The quantification of average T-T signal confirmed that 43% damage removed by 24h from normal GMU6 cells was significantly more than 27% damage removed by PJ-34 treated cells (n=4-7, P<0.05). Next, we examined the effect of PJ-34 on the capacity of hTert-immortalized BJ-EH2 human foreskin fibroblasts to remove UVC-induced T-T and 6-4PP lesions up to 63h and 6h, respectively (Fig. 3.1A, middle and right panels). Unlike normal BJ cells that removed all the T-T signal by 63h, PJ-34 treated cells removed significantly less (50%) damage, which was further confirmed by quantifying average T-T signal from multiple assays (n=6, P<0.05). For removal of 6-4PP lesions, while the final repair by 6h was not affected, the early phase of removal of damage at 1h was significantly suppressed by PJ-34; and quantification of signal confirmed that 45% of damage removed by normal BJ cells was significantly more than 20% damage removed by PJ-34 treated cells (n=3, P<0.01). Lastly, the removal of UVB-induced T-T from epidermis of SKH-1 hairless mice was also reduced up to 48h by PARP inhibitor 1,5-dihydroxyisoquinoline (Fig. 3.1B). Thus, PARP inhibitors significantly decreased the efficiency of removal of UV-induced DNA photolesions in multiple models.
PARP-1 depletion decreases efficiency of removal of UV-induced DNA damage
PARP inhibitors affect activity of all the members of PARP family, hence we examined whether the effect of PARP inhibition on repair of UV-damaged DNA was due to its effect on PARP-1 or on other PARPs. We used GMSiP human skin fibroblasts, in which PARP-1 has been stably and significantly depleted by shRNA without affecting the expression of PARP-2 [372]. We assessed their capacity to: (a) form PAR in response to UVC; and (b) repair the UVC-induced T-T lesions. In the pADPr-immunoblot, a strong signal for heterogeneous bands of PARylated proteins above 116 kDa [373] could be seen in the matched PARP-1 replete GMU6 cells but not in the GMSiP cells (Fig. 3.1C), confirming that PARP-1 is the major, if not the only producer of pADPr in UV-treated cells. Using flow cytometry assay, we noted a marked failure of GMSiP cells to remove T-T from 15 min to 24h (Fig. 3.1D), which is in stark contrast to a significant repair seen in GMU6 cell (Fig. 3.1A, left panel). To exclude the possible differences in the cell cycle phases influencing the repair capacity of these cells [371], we synchronized GMU6 and GMSiP cells in G1 phase and compared their time-course of removal of T-T up to 24h by flow cytometry (Fig. 3.1E) and immunofluorescence microscopy (Fig. 3.1F). By both the techniques, we observed that PARP-1-depleted cells were inefficient at removal of T-T damage. The quantification of T-T signal from flow-cytometry assays confirmed that while GMU6 cells removed 54% of the initial damage, GMSiP cells barely removed any damage (~2%) (n=3, P<0.01). The immunofluorescence microscopy confirmed this trend because GMU6 cells removed 58% of the T-T signal per nuclei by 24h as compared to 15% damage removed by GMSiP cells (n>125 nuclei, P<0.01). Since PARP-1 depletion was sufficient to abolish UV-induced PAR synthesis and impair repair of UV-induced DNA damage, similar to that seen in PARP inhibited cells, PARP-1 is likely to be the main PARP implicated in this repair process.
Characterization of UV-induced interaction between PARP-1 and DDB2
We had earlier shown that PARP-1 rapidly binds to UV-damaged DNA in vitro or in UV-irradiated cells, and it is activated to form PAR within seconds after irradiation at the site of DNA damage [338]. Since DDB2, the early GG-NER protein, is also known to translocate very rapidly at the site of UV-damaged DNA [374], we examined by co-immunoprecipitation (co-IP) studies whether DDB2 and PARP-1 interact with each other in the vicinity of UV-damaged chromatin using cellular fractions that represent chromatin-bound proteins (Ch-fraction) rather than the whole cell extract (Fig. S3.1A). The cell fractionation technique to isolate Ch-fraction was validated by confirming the expected UV-induced relocalization of DDB2 to this fraction in both NER-proficient GM637 and NER-deficient XP-C cells (Fig. 3.2A, 4, 8 and 12), although total cellular DDB2 levels remained unchanged before and after irradiation (Fig. 3.2A, 1, 5 and 9). We further confirmed that UV irradiation promoted the recruitment of downstream NER proteins XPC and XPA to the Ch-fraction of GM637 cells, whereas XP-C cells did not relocalize XPA to the Ch fraction (Fig. 3.2A). For the IP studies, we used GMRSiP cells that express FLAG-tagged human PARP-1 (Fig. S3.1B-C). The IP of Ch-extracts (input) of these cells prepared before or 10 min after UVC irradiation with PARP-1 antibody revealed a significant UV-induced association of DDB2 with PARP-1, which was confirmed in an inverse co-IP with DDB2 antibody (Fig. 3.2B, lanes 4 and 8). Mock IPs with control IgGs confirmed specificity of antibody-based IPs (Fig. S3.1D). To determine if the interaction between these two proteins is direct or mediated via DNA, DDB2-IP was carried out in the presence of 200 µg/ml ethidium bromide to loosen the protein-DNA interactions. The failure of ethidium bromide to prevent co-IP of PARP-1 with DDB2 indicated a direct interaction between these two proteins (Fig. 3.2C, and mock control Fig. S3.1E).
To determine the possible role of catalytic activation of PARP-1 in this interaction, the GMRSiP cells were irradiated in the presence or absence of PARP-inhibitor PJ-34. The FLAG-IP of Ch-fractions before or after irradiation confirmed UV-induced interaction of DDB2 and PARP-1 without PARP inhibitor (Fig. 3.2D, DDB2-lane 4). Interestingly, while PJ-34 treatment did not prevent UV-induced accumulation of DDB2 in the input Ch-fraction prior to IP, it significantly suppressed its ability to associate with PARP-1 (Fig. 3.2D, DDB2-lanes 6 and 8). In an independent model of GMU6 cells expressing mycDDB2 (Fig. S3.2A-C), we confirmed by local UVC irradiation (Fig. S3.2D) and co-IP of Ch-fractions with PARP-1 or myc antibodies (Fig. S3.2E) that PARP-inhibitor PJ-34 did not affect early accumulation of mycDDB2 but blocked its co-IP with PARP-1. We examined whether PARP-inhibitor that disrupts the interaction between DDB2 and PARP-1 also affects the departure of DDB2 from the damaged site, which is a necessary step for the continuation of GG-NER [375]. Immunoblotting for DDB2 in the Ch-fractions isolated up to 2h after irradiation revealed that DDB2 levels which accumulated from 5-15 min declined rapidly by 60 min in the normal cells, but remained high until 120 min in PARP inhibited cells (Fig. 3.2E). Thus, although the initial recruitment of DDB2 to UV-induced chromatin is independent of PARP-1, its subsequent direct association with PARP-1 as well as its eventual departure from the damaged site is dependent on the catalytic activity of PARP-1 that would PARylate proteins near the damaged DNA.
DDB2 stimulates catalytic activity of PARP-1 and becomes a target for PARylation
The influence of catalytic activity of PARP-1 on DDB2 prompted us to examine whether DDB2 is PARylated in response to UV (Fig. 3.3A-B). First, the pADPr-IP of GMU6-mycDDB2 cells pulled down more of mycDDB2 after UVC-exposure, indicating that it is either PARylated or interacts with other PARylated proteins (Fig. 3.3A). That DDB2 could be directly PARylated was tested in an in vitro Dot-blot assay, which showed that pADPr could bind to mycDDB2 (Fig. 3.3B, top panel). The binding of pADPr to its known acceptors PARP-1 and XPA [376] served as positive controls. To confirm that the recipient of pADPr was indeed the designated protein in the immunopurified mycDDB2 preparation, we ran a SouthWestern type of blot in which immunopurified mycDDB2 and purified GST-DDB2 were resolved on SDS-PAGE, blotted and reacted with pADPr (Fig. 3.3B, bottom panel). The mycDDB2 and purified GST-DDB2 displayed one major pADPr-accepting band at 50 or 75 kDa, which corresponds to their respective bands in the DDB2 immunoblots. A strong signal for PARylated PARP-1 at 113 kDa was a positive control, and lack of pADPr binding by proteinase K (Fig. 3.3B) or DNase I and anti-myc antibody (Fig. S3A) served as negative controls. Thus, DDB2 is an acceptor of pADPr.
We next examined whether interaction of DDB2 with PARP-1 has any influence on the activity of PARP-1. In an in vitro PARP-1 activation assay [373], we first observed using bovine PARP-1 and non-labeled NAD (Fig. S3.3B) or immunopurified human FLAG-PARP-1 and biotinylated NAD (Fig. S3.3C) that the catalytic activation of PARP-1 by UV-damaged DNA was stimulated in the presence of mycDDB2, as seen from a heterogeneous smear above 113 kDa for PARylated PARP-1. Moreover, the effect of DDB2 on stimulating PARP-chart). The PJ-34 treatment abolished this additional pADPr synthesis. Collectively these results indicate that DDB2 stimulates the catalytic activity of PARP-1 by UVC-damaged DNA and in turn, PARP-1 PARylates DDB2.
XPC and the interaction of PARP-1 with DDB2
To determine whether XPC that is known to co-IP with DDB2 [146] plays a role in PARP-1 and DDB2 interaction, we used XP-C cells because they could recruit DDB2 to the damaged DNA but not XPA and other downstream GG-NER proteins due to non-functional XPC (Fig. 3.2A). Since PARP-1 activation and pADPr synthesis played a key role in DDB2-PARP-1 interaction, we first examined whether XP-C cells could make pADPr in response to UVC. The time course of pADPr immunoblot revealed that the appearance and extent of PARylated proteins in UV-irradiated XP-C and NER-proficient GM637 cells was comparable (Fig. 3.4A). Using co-IP studies in XP-C cells for PARP-1 (Fig. 3.4B) or pADPr (Fig. 3.4C), we observed that UVC-irradiation promoted an interaction between PARP-1 and DDB2 (Fig. 3.4B) and caused direct or indirect PARylation of DDB2 (Fig. 3.4C). Since these interactions were not as robust as in normal cells (Fig. 3.2B-C and Fig. 3.3A), our results indicate that there may be an XPC-dependent and independent interaction between PARP-1 and DDB2.
Since PARP inhibitor disrupts the interaction between DDB2 and PARP-1 and blocks the departure of DDB2 from the UV-damaged DNA, we determined whether it would also affect the downstream actions of DDB2, such as its interaction with XPC or modification and stabilization of XPC to UV-damaged DNA [146, 367]. Using DDB2-IP of GMU6 cells, we observed that PJ-34 significantly suppressed UV-induced association of DDB2 with XPC (Fig. 3.4D, lanes 6 and 8). Moreover, a slowly migrating heterogeneously modified XPC, often characterized as ubiquitinated XPC [146], was significantly increased in response to UV and suppressed in the PJ-34 treated cells (Fig. 3.4D, lanes 6 and 8). A possible consequence of this effect of PARP inhibition was evident on the recruitment and stabilization of XPC at the site of DNA damage after local UVC irradiation. In the normal GMU6 cells, the signal for XPC colocalized with subnuclear T-T by 10 min and sharply intensified by 30 min. In the PJ-34 treated cells, XPC was weakly localized with T-T at 10 min, and failed to focus properly by 30 min (Fig. 3.4E). The quantification of XPC and T-T in the subnuclear spots revealed that PARP inhibition significantly reduced the colocalization of XPC with T-T (Fig. 3.4E, chart). This was further confirmed by effect of PARP inhibitor on the presence of XPC in Ch-fractions of UV-irradiated GMU6 cells (Fig. 3.4F). In the normal cells, the XPC levels gradually declined from the peak values at 5-15 min up to 4h, whereas in PARP inhibited cells, XPC levels declined rapidly by 30-60 min. In addition, the band for modified XPC was significantly increased from 5-15 min in the normal cells but not in the PJ-34 treated cells (Fig. 3.4F). Thus, PARP inhibition disrupted the interaction of XPC with DDB2 and decreased its stabilization at the lesion site.
Discussion
We show that in response to UV irradiation, PARP inhibited cells have: (a) an impaired capacity to remove UV-induced DNA photolesions; (b) a decreased level of interaction of DDB2 with XPC or PARP-1; (c) an increased tendency for DDB2 to persist at the UV-damaged chromatin; and (d) a decreased level of recruitment, modification and localization of XPC to the damaged site. In addition, we show that: (e) DDB2 and PARP-1 directly interact in the vicinity of the DNA lesion; (f) DDB2 stimulates catalytic activity of PARP-1; and (g) DDB2 is modified by PAR. Our results strongly indicate that PARP-1 is the principle player in above responses, because cells specifically depleted of PARP-1 do not form detectable amounts of PARylated proteins in response to UV, and are also inefficient at repair of UV-damaged genomic DNA. Our results are consistent with earlier reports that impaired PARP-1 function increases UV-induced skin cancer in mice [377], decreases cellular capacity to repair UV-induced DNA damage from viral reporter gene [378] or genomic DNA of CHO or triple negative breast cancer cells [345, 370], and decreases the clonogenicity in response to UV [378].
Our results together with previous reports suggest several possible ways in which PARP-1 can collaborate with DDB2 to increase the efficiency of GG-NER. (i) The PARylated PARP-1 or free PAR chains could serve as a scaffold on which PARylated DDB2 can interact with XPC, such as that suggested for their role with XRCC-1 in the base excision repair [379]. Hence, PARP inhibitor or absence of PARP-1 could reduce participation of XPC in NER. (ii) The catalytic activation of PARP-1 and resultant PARylation of DDB2 could promote chromatin remodelling by DDB2. In fact, PARP inhibitor or PARP-1 depletion was recently shown to block chromatin remodelling of UV-damaged chromatin [186]. In addition, the departure of DDB2 from the UV-induced lesion site was shown to be dependent on chromatin remodelling [177], hence our result showing a delayed departure of DDB2 from UV-damaged chromatin in PARP inhibited cells supports an argument that catalytic function of PARP-1 plays a role in DDB2-mediated chromatin remodelling. This would be in agreement with the reported role of PARylated ALC-1 or APLF-1 in stimulating their chromatin remodelling activity in DNA repair [380]. (iii) The catalytic activity of PARP-1 and PAR formed locally around the lesion could be involved in the ubiquitination activity of UV-DDB-E3 ligase complex, just as PARylation of targets has been shown to facilitate their modification by ubiquitin E3 ligase RNF146/ Iduna [379]. The ubiquitination of different proteins by UV-DDB-E3 ligase produces varying end-results, e.g., ubiquitination of its own members DDB2, cullin 4A, and Rbx1 leads to a disengagement of the ligase complex from the damaged site; that of the histones H2A, H3 and H4 leads to chromatin remodelling [177, 381]; and that of XPC helps in its recruitment and stabilization at the damaged site [381]. While previous study has shown chromatin destabilization effect of PARP inhibition [186], here we show its effect on reduced mobility of DDB2 and decreased modification and stabilization of XPC, all of which could be explained by inefficient ubiquitination activity of UV-DDB-E3 ligase complex. (iv) Finally, a strong effect of PARP inhibition in suppression of the initial phase of repair of 6-4PP in BJ-hTert cells could be due to specific influence of PARP-1 activation on NER at a given site of chromatin, because recently ubiquitination of XPC was shown to promote its relocalization from intra- to inter-nucleosomal region to prioritize the repair at the latter site [367].
Based on our results and previous work, we propose a model for the role of PARP-1 and DDB2 at the lesion recognition step of GG-NER (Fig. 3.5). Immediately after UV irradiation, PARP-1, due to its sheer abundance and known capacity to be rapidly activated in response to different types of DNA damage [368], is likely to be one of the first proteins to arrive at the lesion and be basally activated within seconds of DNA damage (step 1). The arrival of DDB2, which occurs in a similar time frame as PARP-1, will cause a stronger activation of PARP-1, which will result in PARylation of many proteins including PARP-1 and DDB2. This will foster a direct association of DDB2 and PARP-1 that resists separation by ethidium bromide during IP (step 2). Since both proteins are known to independently bind to DNA containing CPD lesions, it is likely that these proteins would interact with the damaged DNA as well as with each other (step 2). One possible scenario to explain the downstream events is that the PARylated DDB2 as part of UV-DDB-E3 complex will remodel the chromatin (step 3A), ubiquitinate DDB2 to reduce its affinity for the lesion (step 3B) and ubiquitinate XPC to promote its stabilization at the damaged site (step 4), which will result in an efficient GG-NER (step 5). As indicated in the model, many of the steps in this model have been shown by us and others [186], implying the role for PARP-1 in collaboration with DDB2 at the lesion recognition step to improve the efficiency of mammalian GG-NER. It will be interesting to examine if PARP-1 also plays other roles in NER.
|
Table des matières
Chapitre 1 Introduction
1.1 Les rayons ultraviolets
1.2 Les dommages à l’ADN induits par les UV
1.2.1 Les dommages directs à l’ADN
1.2.2 Les dommages indirects à l’ADN
1.3 La réparation des dommages induits par les UV
1.3.1 La réparation des dommages indirects par excision de bases (BER)
1.3.2 La réparation des dommages directs par excision de nucléotides (NER)
1.3.2.1 La phase de reconnaissance des dommages
1.3.2.2 La phase de vérification des dommages et la formation du complexe ouvert
1.3.2.3 La phase d’incision du dommage et de synthèse du nouveau brin
1.3.2.4 La phase de ligation du nouveau brin
1.4 La phase de reconnaissance des dommages de la GG-NER
1.4.1 Un détecteur versatile : la protéine Xeroderma pigmentosum C
1.4.2 Un complexe multifonctionnel : le complexe UV-DDB
1.5 La poly(ADP-ribos)ylation (PARylation)
1.5.1 Un « écrivain » : la poly(ADP-ribose) polymérase 1
1.5.1.1 La structure de la PARP1
1.5.1.2 Le mécanisme d’activation de la PARP1
1.5.2 Les « éditeurs » : protéines qui dégradent les polymères d’ADP-ribose
1.5.3 Les « lectrices » : protéines acceptrices de polymères d’ADP-ribose
1.5.4 La PARP1 et la réparation des dommages à l’ADN
1.5.4.1 La PARP1 et le remodelage de la chromatine
1.5.4.2 La PARP1 et la réparation des bases endommagées et des cassures simple-brin
1.5.4.3 PARP1 et la réparation de cassures double-brin
1.5.4.4 La PARP1 et la réparation par excision de nucléotides
1.5.4.5 La PARP1 et la thérapie du cancer
Chapitre 2 Contexte et objectifs de la recherche
Chapitre 3 Role of poly(ADP-ribose) polymerase-1 in the removal of UV-induced DNA lesions by nucleotide excision repair
3.1 Avant-propos
3.2 Résumé
3.3 Abstract
3.4 Introduction
3.5 Results
3.6 Discussion
3.7 Figures and legends
3.8 Materials and Methods
3.9 References
Chapitre 4 Characterization of the interactions of PARP-1 with UV-damaged DNA in vivo and in vitro
4.1 Avant-propos
4.2 Résumé
4.3 Abstract
4.4 Introduction
4.5 Results and discussion
4.6 Figures and legends
4.7 Material and methods
4.8 References
Chapitre 5 Poly(ADP-ribose) polymerase 1 escorts XPC to UV-induced DNA lesions during nucleotide excision repair
5.1 Avant-propos
5.2 Résumé
5.3 Abstract
5.4 Significance Statement
5.5 Introduction
5.6 Results
5.7 Discussion
5.8 Figures and legends
5.9 Materials and methods
5.10 References
Chapitre 6 Discussion générale
6.1 L’absence ou l’inactivation de la PARP1 retarde la réparation des dommages directs induits à l’ADN par les UVC
6.2 La PARP1 coopère avec le complexe UV-DDB pour faciliter la reconnaissance des dommages induits par les UV
6.2.1 Le rôle dépendant de la PARP1 du complexe UV-DDB dans le remodelage de la chromatine
6.2.3 Les rôles de la PARP1 dans les fonctions du complexe ubiquitine ligase UV-DDBCul4A- Rbx1
6.2.3 Le recrutement de la PARP1 aux dommages directs induits par les UV
6.3 Le rôle de la PARP1 dans le recrutement de la XPA
6.4 Le rôle direct de la PARP1 dans la fonction de recherche des dommages de la XPC
6.5 Conclusions
6.6 Perspectives futures
Bibliographie
Annexes
![]() Télécharger le rapport complet
Télécharger le rapport complet