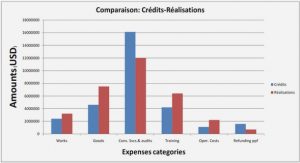
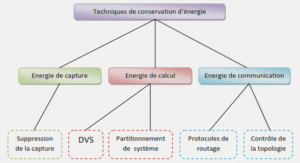
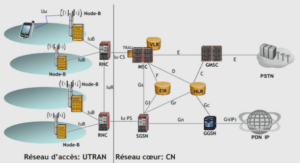
Precipitates in Al-Si cast alloys
Due to the complexity of elements in Al-Si alloy, different precipitates can form, and the section describes several common precipitates in Al-Si cast alloys, and their strengthening effect of Al-Si alloys.
Mg2Si precipitates
Mg2Si precipitate is one of the very common precipitates in Al-Si alloys if Mg is also present in the alloy. The supersaturated solid solution decomposes to form the metastable precipitates designated GP zone, β », β’ until equilibrium β. The precipitation sequence is generally described as follows [63], solute clusters → GP zones (spherical)→β »(needle)→β'(rod)→β. The typical size of needle β » is around 4 * 4 *50 nm3 [64]. Further overageing transforms the needle β » into thick rods β’. The β’ phase forms as rods of approximately 10 * 10 *500 nm3 [65]. The β equilibrium phase is found to be plates with dimensions of several micrometers with composition Mg2Si [66].
The typical TEM micrograph of β » and β’ precipitates and their corresponding Selected area diffraction pattern (SADP) are shown in Fig. 2.10 and Fig. 2.11 respectively [67]. As is seen, they are coherent or semicoherent with Al matrix, thus the strenghening effect can be obtained. In Al-Si alloys with Cu, Mg additions, the optimal strength is often achieved through strengthening of various types of precipitates. Ouellet et al. [68] reported a hardening peak caused by the cooperative precipitation of Al2Cu and Mg2Si phases in Al–Si–Cu–Mg alloys. It has been found that the type of precipitation in the Al–Cu–Mg–Si alloys depends on Mg contents as well as on the Cu:Mg ratio in solid solution. For a Cu:Mg ratio of 2.0 (at. %), preferential precipitation of Mg2Si occurs, while a ratio close to 8.0, promotes the formation of the Al2Cu compound [69]. The increase of Mg content in solid solution contributes to the precipitation phenomenon since clusters of magnesium are the precursors in the formation of Mg2Si in Al–Mg–Si alloys [70].
Besides, metastable Mg2Si precipitates have positive effect on the nucleation of dispersoids. It was reported that α-Al(Mn,Cr,Fe)-Si dispersoids heterogeneously nuclear on β’-Mg2Si in 6xxx alloys [71]. As shown in Fig. 2.12, an intermediate phase u-phase nucleated on the β’-Mg2Si. With continued annealing, α-Al(Mn,Cr,Fe)-Si dispersoids nucleated heterogeneously on the ‘u-phase’ precipitates before these precipitates dissolved.
The precipitation sequence starts with the decomposition of the supersaturate solid solution and the clustering of Cu atoms; the clustering then leads to formation of coherent, disk-shaped GP zones. These zones manifest as two-dimensional Cu-rich disks with diameters of approximately 3-5 nm. With prolonged time, the GP zones increase in number while remain relatively constant in size. If the ageing temperature increase, the GP zones dissolve and are replaced by metastable precipitates θ ». This precipitate is a three-dimensional disk-shaped plate having an ordered tetragonal arrangement of Al and Cu atoms; θ » also appears to nucleate uniformly in the matrix and is coherent with matrix. As ageing proceeds, the θ » starts to dissolve, and θ’ commences to form by nucleating on heterogeneously on dislocations or other defects [75] (Fig. 2.14b). θ’ also has a plate-like shape and is composed of Al and Cu atoms in an ordered tetragonal structure [23, 57]. However, θ’ precipitates grow on further ageing by diffusion of atoms from the supersaturated solid solution to the precipitates [76]. The precipitates continue to grow in accordance with Ostwald ripening [77]. The larger precipitates coarsen as the smaller ones dissolve. As the precipitates grow, the coherency strain increases until interfacial bond strength is exceeded, and the precipitates become non-coherent. So last to form is the non-coherent equilibrium phases (θ), which is completely incoherent with the matrix. In fact, combined with its relatively large size and coarse distribution, the formation of θ reduces the strength significantly [57]. It should be noted that the best strengthening is obtained when θ » is the main strengthening phase.
dispersoids in Al-Si alloys
The dispersoids formed in Al-Si alloys vary with many factors, including alloy composition, thermal treatment etc. The α-dispersoids are the most common dispersoids formed in Al-Si alloys. This section deals with the α-dispersoids that formed in Al-Si alloys with Mn and/or Mo addition. α-Al(Mn,Fe)Si dispersoids can precipitate when Mn is also present. Mn atoms can dissolve in the Al matrix during the solidification, forming a supersaturated solid solution. During homogenization, α-Al(Mn,Fe)Si dispersoids precipitate from the Al matrix. Study [82] investigated the precipitation behaviour of α-Al(Mn,Fe)Si dispersoids and their effect on mechanical properties during precipitation treatment.
Fig. 2.17 shows the precipitation of dispersoids in 3xxx alloy treated at 375 °C for 24 h and their corresponding SADP, and a significant dispersion strengthening effect caused by the precipitation of fine uniformly distributed α-Al(Mn,Fe)Si dispersoids during precipitation treatment is achieved [82].
In addition, α-Al(Mn,Fe)Si are confirmed to heterogeneously nucleated on the pre-precipitated Mg2Si needles [24, 71, 72], leading to the large number density in the matrix. The diffraction pattern of α-Al(Mn,Fe)Si dispersoids are also shown in Fig. 2.18 which is confirmed to be coherent with the matrix [83]. The size of the α-Al(Mn,Fe)Si is reported to be in the range of 60-100nm [24, 71, 72]. Thus, pronounced strengthening can be achieved via formation of fine and large number density of α-Al(Mn,Fe)Si dispersoids [84].
Mo is a transition metal of the sixth group in the periodic table, which is traditionally used as an alloying element for high-strength stainless steels [85].
However, it has not received much attention regarding Al alloys, except few applications in the rapid-solidification powder metallurgy for elevated temperature properties [85-88]. Al–Mo binary phase diagram (Fig. 2.19) shows Mo can promote many AlxMo phases during solidification [89-92]. Mo is also reported to possess low diffusivity in Al [51], making it a potential dispersoid former.
Mo has been applied in Al–Si 356 cast alloy to promote Mo-containing dispersoids [51, 93]. Mo addition helps to form thermally stable nano-scale α-Al(Mo,Fe)Si dispersoids (Fig 2.20), which leads to higher mechanical properties at elevated temperature [51]. Farkoosh et al. also employed combined addition of Mo and Mn in one Al–Si–Cu–Mg cast alloy to form large volume of thermally stable α-Al(Mn,Mo,Fe)Si dispersoids, resulting in more uniform distribution of α-Al(Mn,Mo,Fe)Si dispersoids and elimination of dispersoid free zone (DFZ), due to the opposite partitioning of the Mo and Mn solute atoms during solidification (shown in Fig. 2.21) [93]. In summary, Mo can form a large amount of metallurgical stable dispersoids in Al–Si cast alloys even when added at low levels [51, 92, 94, 95], make it a promising element as α-Al(Mn,Mo,Fe)Si dispersoid former. These dispersoids are thermally stable, hence, significant enhancement in mechanical properties at elevated temperature including the strength and creep resistance was achieved [51, 72, 93].
Fig 2.20 (a) Bright field TEM micrograph showing the Al(Mo,Fe)Si dispersoids in the interdendrite regions of the MG3R3M alloy formed after 10 h of solution treatment at 540 °C. (b) EDS spectrum of the Al(Mo,Fe)Si dispersoids.
Creep behaviour and its mechanism
Creep is a permanent deformation of materials under constant load and at constant temperature. It can occur as a result of long-term exposure to high levels of stress that are still well below the yield strength of the material.
Creep stages
Generally, for tensile creep test, there are three different stages as shown in Fig.
The primary creep stage is characterized with very high strain rate due to the
material elastically and plastically responds to the applied load. As the deformation continues, the material is continually strengthened by work hardening, which leads to the gradual decrease of the creep rate. As the deformation continues, primary creep stage gradually transits into the secondary creep stage. This stage is also called the steady-state creep. The creep rate remains nearly unchanged for a prolonged period under a constant load. This is an interplayed result between recovery and hardening.
The secondary creep region usually dominates most of the time of creep deformation.
As creep proceeds, the secondary creep changes into the third stage (tertiary creep).
Tertiary creep only occurs in tensile creep test. As continuous deformation produces voids or internal cracks which decrease the cross-section hence increase the stress. As a result, a necking occurs at tertiary stage of the creep, which ends up the fracture of the materials (Fig. 2.22). The tertiary stage creep possesses a much higher creep rate.
For compression creep curves, there is no such necking as occurred in tensile creep tests, due to the geometric effect that the sample cross-section swelled as the deformation continues. Thus, the steady-state creep stage dominated during compression creep. As shown in Fig. 2.22 (dotted lines), no tertiary creep can be observed during compression creep test.
Parameters of creep properties
Many researchers [96, 97] proposed many equations to evaluate creep properties of metals and alloys. These equations summarized the total strain and time dependent creep rates. When the creep strain and temperature are lower, the creep strain [98] canbe expressed as:
Strategies to improve creep resistance
The creep resistance of materials could be improved by the following methods
1. Dispersoids are effective for improving the creep resistance as well because they are usually thermally stable at elevated temperature. Besides, their coarsening rate is lower than precipitates particles.
2. Solid solution hardening is also beneficial to creep resistance. This is best achieved by use of solutes differing markedly in atom size and valence from the parent metal. Long range order in solid solutions provides a further contribution to the creep strength of solid solutions, because the super-lattice dislocations are paired to preserve order across the slip plane and are thus similar to extend dislocations.
3. Precipitates can also increase the creep strength, and a theory provides an estimate of the critical spacing if dispersion for optimum strength in terms of that just small enough to prevent dislocations bowing around the particles. Some precipitates form more readily than others on dislocations, and thus are important source of strengthening, both at low and elevated temperatures. Precipitates which form during creep are particularly useful if they nucleate on dislocations.
4. Creep resistance is greater in a matrix of low stacking fault energy, because the dislocations are dissociated, and thus more difficult to cross-slip and to climb, in order to avoid obstacles. The stacking fault energy of a pure metal can be lowered by solute additions. For this purpose, solutes of high valence are best because they more readily increase the electron/atom ration, and thus decrease stacking fault energy.
Thermal exposure behaviour of Al–Si alloys
Piston operation induces a complex combination of thermal stresses and high cycle, high temperature mechanical cycles in the material. The typical working temperature of these parts can reach up to 250oC-300oC, as is shown in Fig. 2.24.
For Al-Si alloys, the mechanical properties often deteriorate with increasing thermal exposure at 200 oC-300 oC. Hardness-time-temperature (HTT) curves can be applied to evaluate their thermal stability, as they offer an immediate overview of the mechanical response after thermal exposure [109], as such experimental data are useful for validating the simulations of piston temperature distribution. Fig. 2.25 presents the HTT curve of Al-Si 4032 alloy after forging and T6 heat treatment. The microhardness decreases with increasing temperature and increasing time. Moreover, the degree of degradation is greatly related to the thermal exposure temperature.
When temperature is lower than 200oC, the hardness remains relatively stable even with prolonged exposure. However, when temperature exceeds 200oC, the higher the temperature, the faster the decreasing of mechanical properties.
As discussed in previous sections, the precipitates and dispersoids are crucial to the mechanical properties of Al–Si alloys. They can precipitate during a variety of thermal treatments, such as ageing or annealing process thus strengthening Al-Si alloys. During prolonged thermal exposure, the precipitates will experience certain degree of coarsening, leading to the deterioration of mechanical properties. Thus, it is important to study the kinetics of precipitates/dispersoids under thermal exposure.
This has special practical importance for Al–Si alloys designed for prolonged thermal exposure.
Coarsening of precipitates
When precipitation from supersaturated solid solution is completed, prolonged treatment leads to precipitate coarsening driven by the interfacial free energy between the precipitate and the matrix (Ostwald ripening). The physical process by which the microstructure coarsens and releases its excess surface energy is due to the higher solubility of small particles, since these have a larger ratio of surface area to volume.
The larger particles thus grow at the expense of the smaller ones, and the growth kinetics depend upon the rate-controlling step in the process. Those particles which have a radius < r∗ (critical particle radius) will have a negative growth rate according to equation and will thus shrink. When the size of a group of particles reaches zero, they are removed from the size distribution.
Here, Dγ is the diffusivity of elements in Al matrix, Vm is the precipitate-matrix interfacial free energy, and Ce is the equilibrium solubility (assuming a planar interface) of the solute species in the precipitate and matrix phases, respectively.
For any thermal resistant alloy, it is essential that the dispersoids/precipitates resist coarsening during prolonged exposure at elevated service temperatures. This isespecially imperative for Al–Si alloys because of the generally limited solubility of most solutes in Al and the concomitant limited volume fractions of dispersoid phases.
For Al-Si alloys, the precipitates experience coarsening during prolonged thermal exposures. As mentioned in previous sections, in Al–Si alloys with Cu addition, Al2Cu is the main strengthening phases. During ageing, GP zone, θ », θ’ and θ can precipitate.
The GP zones and the matrix have the same crystal structure. The transition metastable phases θ » and θ’ are less stable than the equilibrium θ phase. Fig. 2.26 listed the solvus temperature of all Cu metastable and stable phases.
Coarsening of dispersoids
Coarsening of dispersoids is also very common in alloys with dispersoids as the main strengthening phase under thermal exposure. The mechanism of coarsening of dispersoids can also be accounted by Ostwald ripening. However, the dispersoids can retain better stability at prolonged thermal exposure due to their relatively higher precipitation temperature than precipitates. Fig. 2.30 shows the evolution of α-dispersoids during long thermal exposure at two different temperatures, 350 oC and 400oC respectively. It is evident that the α-dispersoids generally exhibit good thermal
stability compared with precipitates discussed in section 2.5.1. The α-Al(Mn,Mo,Fe)Si dispersoids (Fig. 2.30b and Fig. 2.30d) shows improved thermal stability than α-Al(Mn,Fe)Si (Fig. 2.30a and Fig. 2.30c) at identical thermal exposure condition characterized with less diameter increase. The improved thermal stability of α-Al(Mn,Mo,Fe)Si is related to its lower coarsening rate than α-Al(Mn,Fe)Si, and this can be ascribed to the Mo’s four order’s magnitude less of diffusion rate than Mn.
Intermetallics changes during thermal exposure
In addition to the coarsening of precipitation and dispersoids, factors that should be considered for Al-Si alloys with thermal exposure at elevated-temperature include: intermetallics changes and synergistic effects of above-mentioned mechanisms [116].
One good example is the coarsening of eutectic Si particles in Al-13%Si piston alloys.
The eutectic Si particles experience continuous fragmentation and spheroidization during prolonged thermal exposure at elevated-temperatures, consequently, leading to the deterioration of mechanical properties [117-119].
As a matter of fact, the deterioration of mechanical properties under prolonged thermal exposure often involves with the synergistic influence of various factors, hence measures to improve thermal stability in Al-Si cast alloys depend on what properties are important in a particular application [116]. When strength is critical which is often the case in Al-Si alloys, coarsening of the matrix precipitates during elevated-temperature service will be important, thus providing extra strengthening
phases or retardation of coarsening process would be reasonable methods to improve its thermal stability. In conclusion, the deterioration of mechanical properties might be universal during prolonged thermal exposure, but the exact mechanisms and measures to improve the thermal stability should be dealt with extra prudence in certain circumstances.
Property measurements
Microhardness
Microhardness was measured on polished sample by Vickers hardness tester at room temperature to evaluate the room-temperature properties. The tests were conducted by NG-1000 CCD microhardness test machine with a load of 10 g and a dwelling time of 20 s. A total of 15 measurements were performed for each sample, and the average was considered to be the hardness of each sample. During the hardness tests, the indentations were guaranteed inside the dendrites without touching the intermetallics formed during the solidification in order to show the evolution of hardness from the Al matrix.
Yield Strength
The compression yield strength test was performed by Gleeble 3800 thermo mechanical testing unit. They were tested at both room temperature (25 oC) and elevated-temperature (300 oC) with the fixed strain rate of 0.001 s-1. The samples were machined into cylinder with a 15mm length and 10mm diameter before compression tests. They were heat treated at different temperatures for different periods of time before testing. Conditions selected for the tests can be found in Chapters 4, 5 and 6.
The average value obtained from three samples was calculated to be the yield strength of each sample.
Creep resistance
The compressive creep tests were performed at 300 oC for 96 hours. The samples were machined into cylinder with a 15mm length and 10mm diameter. The data was recorded and further analyzed. For each test, total creep strain and minimum creep rate were calculated to evaluate their creep resistance. Creep load should be carefully selected depending on the sample’s elevated-temperature yield strength, which is fixed to 60-70% of its yield strength.
Microstructure characterization
Optical microscopy (OM)
Optical microscope (Nikon Eclipse ME600) was used to examine the microstructures of experimental samples in as cast and thermal treated conditions. The samples were sectioned, mounted and metallographically polished. In addition, the image analyzer (CLEMEX JS-200, PE4.0) was used to characterize the Fe-rich intermetallics, dispersoids as well as Si particles, such as the size and aspect ratio. In order to show the distribution of dispersoids and the dispersoid free zone, some selected sample were etched in 0.25 vol. % HF solution.
Scanning electron microscopy (SEM)
A scanning electron microscope (SEM, JSM-6480LV) equipped with energy-dispersive X-ray spectroscopic (EDS) facilities was used to identify the Fe-rich intermetallics in the as-cast samples and quantify the alloying elements in the intermetallics. In addition, SEM was also used to observe the Si particles in both as cast and thermal treated samples, after specimens were deep etched in 20 vol.% NaOH solution for 20 min.
Electron backscatter diffraction (EBSD)
For more accurate phase identification, EBSD was applied to identify the Fe-rich intermetallics by comparing simulated EBSD pattern with standard EBSD patterns of corresponding phases and their mean angular deviation (MAD) value. The maximum value of MAD value 0.7 is accepted for the accurate phase identification [4].
|
Table des matières
Abstract
Résumé
Acknowledgements
Publications
Table of Contents
List of Tables
List of figures
Chapter 1 Introduction
1.1 Background
1.2 Objectives
References
Chapter 2 Literature Review
2.1 Introduction of Al-Si alloys
2.2 Microstructure of Al-Si cast alloys
2.2.1 Main intermetallics in Al-Si alloys
2.2.2 Fe-rich intermetallics
2.3 Strengthening mechanisms at elevated temperature
2.3.1 Solid solution strengthening
2.3.2 Precipitation strengthening
2.3.3 Dispersoid strengthening
2.4 Creep behaviour and its mechanism
2.4.1 Creep stages
2.4.2 Parameters of creep properties
2.4.3 Creep mechanism
2.4.4 Strategies to improve creep resistance
2.5 Thermal exposure behaviour of Al–Si alloys
2.5.1 Coarsening of precipitates
2.5.2 Coarsening of dispersoids
2.5.3 Intermetallics changes during thermal exposure
References
Chapter 3 Experimental
3.1 Alloy design and sample preparation
3.2 Heat treatment design
3.3 Property measurements
3.3.1 Microhardness
3.3.2 Yield Strength
3.3.3 Creep resistance
3.4 Microstructure characterization
3.4.1 Optical microscopy (OM)
3.4.2 Scanning electron microscopy (SEM)
3.4.3 Electron backscatter diffraction (EBSD)
3.4.4 Transmission electron microscopy (TEM)
References
Chapter 4 Evolution of Fe-rich intermetallics in Al–Si–Cu 319 cast alloy with various Fe, Mo, and Mn contents
4.1 Introduction
4.2 Materials and Methods
4.3 Results and Discussion
4.3.1 Precipitation of Fe-rich Intermetallics in experimental alloys
4.3.2 Evolution of Fe-rich intermetallics with Mo and Mn additions
4.4 Conclusions
References
Chapter 5 Evolution of dispersoids and their effect on elevated-temperature mechanical properties and creep resistance in Al–Si–Cu 319 cast alloys with Mo and Mn additions
5.1 Introduction
5.2 Materials and Methods
5.3 Results and discussion
5.3.1 As-cast microstructure and properties
5.3.2 Evolution of Dispersoids during heat treatment
5.3.3 Role of dispersoids on YS at RT and elevated temperature
5.3.4 Evolution of creep resistance with Mn or/and Mo additions
5.3.5 Long-term thermal stability of elevated-temperature properties
5.4 Conclusions
References
Chapter 6 Improved elevated-temperature properties in Al-13%Si piston alloys by Mo addition
6.1 Introduction
6.2 Materials and Methods
6.3 Results and discussion
6.3.1 As-cast microstructure of experimental alloys
6.3.2 Dispersoid evolution during heat treatment at 520 oC
6.3.3 Yield Strength at room and elevated temperatures
6.3.4 Evolution of properties after thermal exposure at 300 oC
6.4 Conclusions
References
Chapter 7 Conclusions and Recommendations
7.1 Conclusions
7.2 Recommendations
![]() Télécharger le rapport complet
Télécharger le rapport complet