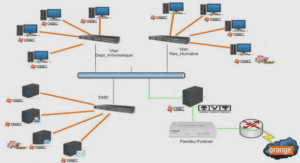
Identification of resinic acids using GC/MS Analysis
Introduction
In North America, following the massive antibiotherapy that occurs in 1980fs, resistance for certain antibiotics was developed. Thus, when time comes to treat an infection, serious problems happened. The sensitive bacteria were eliminated, but the resistant ones were selected. Among these organisms, methicillin-resistant Staphylococcus aureus (MRSA) has been found tohave a major impact on patient care and have been responsible for numerous outbreaks in hospitals. The prevalence of MRSA increased from less than 3% in the early 1980s to rates as high as 40% in many hospitals in the United States and Europe1″4. The Canadian Nosocomial Infection Surveillance Program (CNISP) indicates an increase of 14% MRSA instance only in 2006-20072. Currently, approximately 95% of the strains are resistant to penicillin G (aminopenicillins, carboxypenicillins and ureidopenicillins)3. Whereas the Community strains are, in general,sensitive to the penicillin M (methicilline, oxacillin), but not nosocomials strains3. The glycopeptides remain generally active on MRSA-H5. Since 1997, stocks having sensitivity to the glycopeptides decreased, they are more commonly called GISA (Glycopeptide Intermediate Staphylococcus aureus)5. Currently, the vancomycin, is the antibiotic of choice to treat the infections with MRSA-H. However, considering that several MRSA resistant to the vancomycin (VRSA) were discovered first in Japan (1996) and then in United States (1999), its effectiveness is perhaps not guaranteed for the next years6’7. Since the resistant bacterial infections are in the process of becoming major public health problems the search for new active antibiotics against the resistant bacteria is a very important task5. In the past 25 years, 68% of antibacterial discovered are from natural origin9. Actually, only about fifty percent of plants were studied for their chemical composition and biological activities. Consequently, it is relevant to assess antibiotic activity from plant extracts and to isolate bioactive compounds.
Pinus banksiana (Jack Pine) is the most widely distributed pine species in Canada, from the Cape Q Breton Island in Nova Scotia up to the river Mackenzie in Territory of the Northwest .
P. banksiana, like others conifers, are subject to attacks by a wide range of pathogens as fungi and bacteria. The principal chemical and physical defence of conifers is made of inducible production of oleoresin10 which possess antifungal and antibacterial activities. Moreover oleoresin was used by the first nation to healed infected wounds, boils and pyodermas11. Conifer buds contains also protective oleoresin. In this study, antibacterial activity of buds extracts from P. banksiana was evaluated for the first time against clinical isolates MRSA and some antibacterial compounds were identified.
Material and Methods
Plant material
Buds of Pinus banksiana were collected in the boreal forest of Saguenay region, Québec, Canada, in May 2007. The specimen was identified by Patrick Nadeau (Département des sciences fondamentales, Université du Québec à Chicoutimi). A voucher specimen (QFA-0540468) was deposited at the Herbarium Louis-Marie of Université Laval, Québec, Canada.
Chemicals
Abietic acid were purchased from Sigma-Aldrich (purity 75%) and neoabietol, dehydroabietic acid, abiet-8-enic acid, palustric acid, pimaric acid, isopimaric acid, levopimaric acid and sandaracopimaric acid were purchased from Helix Biotech corporation (purity >85%).
Extraction and antibacterial-bioguided screening of extracts and fractions.
The air-dried buds of Pinus banksiana (1.6 kg) were reduced in powder before being successively extracted with hexane, CH2CI2 and MeOH using a Soxhlet apparatus (5 L each for 48 h).Evaporation under reduced pressure, at a temperature not higher than 45°C, yielded a hexane extract (489 g), a CH2CI2 extract (84 g) and a MeOH extract (166 g). The bioactive hexane extract was subjected to silica gel columnchromatography using a gradient elution of Hexaneethyl acetate (100:0, 95:5, 85:15, 70:30, 50:50) to give 7 fractions. Bioactive fraction 2 was analyzed by GC/MS.
Identification of resinic acids using GC/MS Analysis
Bioactive fraction 2 was methylated with diazomethane as described by Schlenk and Gellerman,I96012. Analyses by GC-MS were performed on a Agilent Technologies (Model 6890N) gas chromatograph, equipped with a DB-5 column (30 m x 0.25 mm x 0.25 |um) and coupled to a mass spectrometer (Model 5973 Network) equipped with an automatic injector (Model 7683 series). Helium was used as a carrier gas with a flow rate of 1 ml/min and the split ratio was 50:1.The injector temperature was 250°C with a MSD transfer line heater temperature of 280°C. The oven temperature was set to 100°C for 2 min, then raised by 3°C/min to reach 280°C and then was left constant for 10 min. Identification of resinic acids was made on the basis of their retention indices on DB-5 columns13 and their mass spectra, which were compared with resinic acids standard and reference data14.
Isolation and identification of methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) strains.
From March 2007 to March 2008, 35 screening swabs from nares (n=31), throat (n=3), Groin pus (n=l) were obtained from patients hospitalized at Chicoutimi hospital. Identification of S. aureus was performed using the Slidex Staph-Kit (bioMerieux Vitek, Inc., Hazelwood, Mo.) according to the manufacturer’s instructions. Quality control was performed with MRSA strain ATCC 43300 and S. aureus strain ATCC 25923. In addition, S. aureus profile was detemined with API Staph test strip with bacterial suspension of saline McFarland of 0.5 (bioMerieux, Durham, NC) according to the manufacturer’s protocol. API Staph strips were held for 24 h before the results were read. A latex agglutination (SLIDEX) test detecting methicilin resistance in Staphylococci based on the production of low-affinity PBP2a, which is encoded by the mecA gene was performed on all strains (data not shown). An antibiogram was also performed to confirm the identification of MRSA strains using disk diffusion test.
Antibiotic susceptibility testing using disk diffusion method.
The antimicrobial activity of different classes of antibiotics was determined by diffusion method (Kirby-Bauer) as described by NCCLS. The antibiotics tested included: penicillin (10 units), amoxicillin/clavulanic acid (20/10 jig), ciprofloxacin (5 |ag), moxifloxacin (5 |ig), levofloxacin (5 jag), clindamycin (15 |ig), erythromycin (15 |ig), cefoxitin (30 |ig), linezolid (30 |j,g), trimethoprim/sulfomethoxazole (1.25/23.75 |ig), rifampicin (5 (ig), gentamicin (10 [xg) and vancomycin (30 \xg). The agar medium had pH 7.2 to 7.4 before inoculing potentiel MRSA. The antibiotics were maintained at 8°C or lower -14°C according to manufacturer’s recommendations.
The pure culture was inoculated on a non-selective plate for 18-24 h. Each colony was collected with a wire loop and transfered the growth to a tube containing 4 to 5 mL of a tryptic-soy broth. The inoculum density was standarized with BaSCU using 0.5 McFarland standard.
|
Table des matières
1. INTRODUCTION
1.1 Caractéristique générale de Staphylococcus aureus
1.2 Cause et conséquence de l’infection par Staphylococcus aureus
1.3 Mécanismes de la résistance bactérienne
1.4 Incidence et résistance aux antibiotiques
1.5 Caractéristiques des SARM
1.6 Problématique
1.7 Objectifs du projet de recherche
2. REVUE DE LITTÉRATURE SUR PINUS BANKSIANA LAMB (PIN GRIS)
2.1 Description et distribution
3. ARTICLE 1
3.1 Résumé
3.2 Abstract
3.3 Introduction
3.4 Material and Methods
3.4.1 Plant material
3.4.2 Chemicals
3.4.3 Extraction and antibacterial-bioguided screening of extracts and fractions
3.4.4 Identification of resinic acids using GC/MS Analysis
3.4.5 Isolation and identification of methicillin-sensitive Staphylococcus aureus (MS SA) and methicillin-$ resistant Staphylococcus aureus (MRSA) strains
3.4.6 Antibiotic susceptibility testing using disk diffusion method
3.4.7 Antibacterial activity measurement using microdilution method
3.5 Results and Discussion
3.5.1 Extraction of buds from P. banksiana, fractionation and evaluation of antibacterial activity against S. aureus and E.coli
3.5.2 Chemical composition of bioactive fraction from hexane extract
3.5.3 Isolation and characterisation of clinical isolates MRSA
3.5.4 Evaluation of antibacterial activity of resin acids against MS SA and MRSA.
3.5.5 Acknowledgments
3.5.6 References
4. CONCLUSION ET PERSPECTIVE
5. RÉFÉRENCES
![]() Télécharger le rapport complet
Télécharger le rapport complet