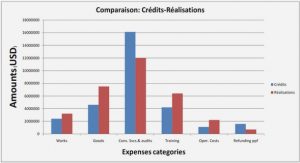
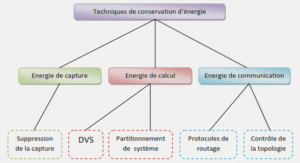
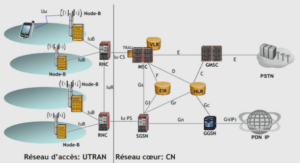
Histology and Skeletal Preparation
Whole-mount specimens of ammocoetes (4 populations, n = 79, TL = 118-154 mm), metamorphosing ammocoetes (2 populations, n = 14, TL = 125-144 mm) and young adults (4 populations, n = 21, TL = 114-153 mm) were c\eared-and-stained (C&S) following Potthoffs protocol [92]. Alcian blue (0.3%) in acid solution was used to color the cartilaginous structures. A structure was considered formed when uptaking the blue stain.Specimens were examined under a Leica MZ16A binocular microscope equipped with a Qicam digital camera with a CCD sensor (Meyer Instruments, TX). To determine the skeletogenesis through the stages, the presence of 46 skeletal elements was observed for ammocoetes and 136 elements for metamorphosing ammocoetes and adults. Each element was coded into a binary database; developmental events occurring in medianfins (e.g., formation of rays, bifurcation of rays) were also coded. Two possible developmental states were observed: (0) absence and (1) presence. Drawings were made using a camera lucida mounted on a Leica MS9.5, then were vectorized with Inkscape (Version 0.48, Free Software F oundation, 1990) and corrected using Adobe Photoshop Element (Version 9.0.3, Adobe Systems Incorporated, 2013). Samples of branchial, predorsal, 02 and caudal regions (see definitions below) of an ammocoete (Old Woman River, AMPMH-Ol , TL = 132.9 mm) and an adult (St. Lawrence River, ADPMH-Ol, TL = 270.5 mm) of P. marinus were selected for histological preparation. Samples were processed in a Shan don Citadel 2000 automated tissue processor (www.thermoscientific.com). They were dehydrated in graded ethanol solutions (20- 100%), transferred to xylene/ethanol and finally pure xylene. Samples were then transferred to a 50/50 xylene/paraffin solution and impregnated with melted paraffin under a vacuum.Samples were embedded in Paraplast Plus (www.mccormickscientific.com) and sectioned at 7 )lm intervals. Sections were processed through regressive staining using standard haematoxylin and eosin (H&E) (http://www.sigmaaldrich.com). Sections were observed with a Leica DMLB microscope and images were taken with an AmScope MUIOOO microscope digital camera using Toupview software (Version 3.7, AmScope, 2013).
Nomenclature and Definitions
Nomenclature for the Sea Lamprey skeleton follows that of Jollie [8], Bardack and Zangerl [93], Hardisty [1], Yao et al. [12], Richardson and Wright [Il], Martin et al. [9] and Richardson et al. [5]. Arcualia are defined as the primary cartilaginous elements forming dorsally along the notochord in vertebrates [51 ,94]. During embryonic development, the first mesenchymal cells gather around the notochord, forming discrete blocks of cartilage [51]. These structures, mainly formed of hyaline cartilage, develop into neural arches in derived groups of fishes [51 ,95,96]. This term is also used to designate the dorsal vertebral elements in the Petromyzontiformes [5,31 ,43,48]. We defined mediodorsal vertebral elements as the cartilaginous structures present alongside arcualia; these elements are attached dorsally to the notochord and positioned inwardly to the arcualia. The development of arcualia was categorized in terms of their position along the body axis. The branchial region (B) starts at the first branchial arch and finishes at the level of the seventh branchial arch inclusively. The predorsal region (P) covers the area from the seventh arch to the first fin ray of the first dorsal fin (D 1). The Dl region (D 1) contains the first to the last ray of the first dorsal fin. The D2 region (D2) is delimited between the first and the last ray of the second dorsal fin; since the second dorsal fin is continuous with the caudal fin, we considered the shortest ray of the posteriormost region of D2 as the limit between D2 and the caudal fin. The caudal region (C) encompasses the last fin ray of the second dorsal fin to the posterior extremity of the notochord. Each region was grossly separated into three zones: (l) anterior, (2) middle and (3) posterior. Development of arcualia was defined as follows: absent if no arcualia is observed (0); weakly developed if arcualia are small cartilaginous nodules that are barely visible along the notochord (+); moderately developed if arcualia are rather sm ail but well visible along the notochord (++) and well developed if arcualia are noticeably long and large (+++).Fin rays are defined as the structures intemally strengthening and supporting the fins. Rays can be formed of cartilaginous, mineralized and osseous tissues. Four kinds of rays have been described to date. Lepidotrichia, present in actinopterygians and sarcopterygians with the exception of dipnoans, are flexible rays composed of successive mineralized hemisegments connected together by ligaments [97,98]. The distal ends are dichotomously branched. Actinotrichia are present between the most distal hemisegments of the lepidotrichia. This kind of ray consists of short, tapered cartilaginous rods that are generally distally branched [97,98]. Actinotrichia can also be found in the adipose fin of some Salmonidae and Siluridae [98]. Ceratotrichia are flexible, unsegmented cartilaginous rods present in the fins of chondrichthyans. Those rays are longer and thicker than actinotrichia and are usually branched distally [97,99, l 00]. Lastly, camptotrichia, present only in dipnoans, consist of straight cylindrical rods arranged in two asymmetrical rows [51,99]. This kind of ray is usually dichotol11ous at the lllargin and is composed of acellular fibrous tissues and mineralized bones [51]. Despite the terms that have been used in previous studies (i.e., radiais) [32] we will use the term fin ray for lampreys in the present study.Staging of metamorphosis was based on morphological traits described by Youson and Potter [20].
Staging
Staging of specimens S3-1 to S3-9 was consistent with the stage 4 of Youson and Potter [20]; these specimens possessed fused lips, an oval-shaped oral disc and small, underdeveloped fimbriae (See Appendix). Specimens S3-10 to S3-14 were c1assified as stage 5 based on the presence of well-developed fimbriae, a slit-like mouth and an elongated snout (see Appendix). PCA provides a descripton of the global interaction among traits (Fig. la). The first principal component (PCI) explains 71.4% of the total variation, whereas PC2 explains 20.1 % (Table 1). PC 1 primarily corresponds to the general growth of metamorphosing ammocoetes, with a clear allometric change of proportion of the mouth (MW, ML). Moreover, traits related to the size of the mouth (MW, ML) contribute for most of the variation on PCI (Fig. la, Table 1). Length traits tend to be grouped and inversely correlated with width traits (MW, IL). The interocular distance (IL), branchiallength (BL) and postocular length (POL) do not contribute to a great deal of variation in PC 1 and PC2. PC2 corresponds to the shape variation (Fig.l 0, Table 1). The mouth width (MW) and the prenostril length (PNL) contribute for most of the variation on PC2, whereas the postocular length (POL) contrasts slightly with the remaining variables on PC2. MDS analysis showed that the classical staging by Y ouson and Potter [20] is not consistent with the staging based on the presence of skeletal elements (Fig. Il). No clusters appeared among stage-4 and stage-5 specimens on the ordination diagram; however, adults (S4) and metamorphosing ammocoetes (S3) are greatly dissimilar, as shown on dimension 1. The longest specimen that belonged to stage 4 (S3 -9) is the only metamorphosing specimen to be greatly dissimilar from other specimens; it is not clustering either with adults or with the other metamorphosing ammocoetes.
Patteming and Morphology of the Appendicular Skeleton
Previous studies have extensively described the skeletogenesis of Petromyzon marinus; the formation of skeletal elements starts at 13 days post-fertilization (dpf) and is achieved around 33 dpf [5,9-11], which corresponds to stage 18 as described by Piavis [10,28). Considering that ail skeletal elements of the ammocoete are present by that stage [7,9,14,43], we assumed that the skeletal development occurring afterwards was limited to somatic growth. However, our data showed that skeletogenesis was still ongoing during the ammocoete stage; the appendicular skeleton develops in a predetermined sequence of seven steps that is size (i.e., total length) dependent. We are the first to demonstrate a direct relationship between the size of an ammocoete and the skeletal development of the median fins. Size was found to be a better predictor than age for skeletal development in numerous osteichthyans [101,102). Since developmental progress and growth respond similarly to environmental variation, body size represents a cumulative measure of both processes [102). The size is therefore a good proxy to discriminate ammocoete since it directly reflects the progression of the development in the median fins. This peculiar relationship was not found in metamorphosing ammocoetes and adults. Our results showed that fin rays of P. marinus developed first in the second dorsal fin (step 2-3), followed by the caudal fin and the first dorsal fin (step 4-5). Fin rays of the first and second dorsal fins always developed anteroposteriorly. These results contrast with the condition in actinopterygians, where the endoskeletal and exoskeletal elements develop first in the caudal fin [84,86,87,89, 101,103]. In these derived groups, bidirectional development of the skeletal elements is the most common pattern found in the dorsal, anal and caudal fins [98,103 ]. In fact, only a few taxa (i.e., Carangidae, Scombridae) are known to possess anteroposterior development at the level of the dorsal fins [103]. However, fin rays of the caudal fin developed bidirectionally starting at the posteriormost region in P. marinus; observations of this highly conserved pattern in P. marinus corroborate the plesiomorphic condition of this condition. Even if the second dorsal fin and the caudal fin are not distinctly separated in P. marinus, their developmental independence is obvious. Our results showed that fin rays were developing in both dorsal fins at different times and did not fo llow the same differentiation pattern. The longitudinal elongation of fins is often the result of the fusion of ail median fins, as seen in many different groups of fishes (e.g., lampreys, hagfishes, pleuracanth sharks, Polypteriformes, Anguilliformes, Ophidiiformes) [103,104]. Such fins are highly specialized and are derived from separate, short-based fins [104];thus, it is normal to observed different differentiation patterns. The fusion between the second dorsal and caudal fin may represent a derived condition and does not necessaril y imply a developmental dependency. Descriptions of the general morphology of the appendicular skeleton in lampreys are scarce in literature. The presence of median cartilaginous rods, bifurcation and posterior fusion of fin rays in the posteriorrnost region of the caudal fin have previously been observed by Tretjakoff [48] Goodrich [32,48] and Jollie [8]. Posterior fusion of the rays occurred at the caudal fin level in step 7 ammocoetes. Jollie [8] described this condition as a skeletal mass enclosing the end of the notochord and the neural and haemal canal, with »fin radiais » extending out from this mass to the margin of the caudal fin. Fusion of skeletal elements in the caudal complex represents an adaptation that has been observed in many osteichthyans: the fusion of modified uroneurals and epurals in the Salmonidae, resulting in the formation of the stegural [77]; fusion of hypurals to form a hypural plate in advanced teleosts (e.g., atherinomorphs, gasterosteids, scombrids) [105,106] and thc urostyle of Perciformes, formed by several urocentra, fused with hypurals elements [107]. In every case, the fusion of posterior elements has a specifie purpose for the propulsive locomotion. However, Petromyzontiformes possess an undulatory swimming and are known to possess a poor propulsive ability [24,108]. We observed that the posterior end of the notochord was slightly curved upward in smaller ammocoetes, whereas more advanced ammocoetes (i.e., step 7) with a posterior fusion possessed a fairly straight notochord. We hypothesise that the function of the posterior fusion in P. marinus might help to increase the lateral stabilisation of the notochord. The morphology of the caudal skeleton of P. marinus is rather similar to that of hagfishes (e.g., Myxine glutinosa, Eptatretus burgeri and Bdellostoma sp.). Ota et al. [70] have recently showed that cartilaginous median rods are present dorsally to the neural tube and ventrally to the notochord in addition to bifurcation in the fin rays in the caudal fin of E. burgeri. In this species, the posterior fusion occurs between both median rods at the posteriormost end of the caudal region, enclosing the tips of the notochord and the neural tube. In the different species of hagfishes for which the information is available (i.e., M glutinosa, E. burgeri and Bd. sp.), the median rods are noticeably larger than the ones found in P. marinus. Since the skeletogenesis of the median fins has never been studied in hagfishes, we cannot determine if the median rods result from the fusion of the proximal ends of the rays, as was seen in P. marinus. Owing to the difficulty of outgroup comparison at this level of the craniate phylogeny, we cannot determine whether the similarity in the caudal skeleton of hagfishes and lampreys constitute shared synapomorphies or symplesiomorphies.
Fin Rays
Lamprey fin rays have sometimes been called « fin radiais » [61,109]. It has been uncertain whether the cartilaginous rods observed in the median fin lobes of lampreys are true rays or fin radiais. Fin rays are endochondral elements intemally supporting and strengthening the fins [32,9 1,97], whereas radiais are defined as endochondral elements linking the median fins of gnathostomes to the axial skeleton [51 ,91]. Fin rays are articulated proximally to the radiais [51]. The fin rays of P. marinus show gross similarities to lepidotrichia; in both cases, the fin rays are present from the base to the border of the fin,thus intemally supporting the fin. Both types of fin rays possess distal bifurcation that results in the emergence of sister rays; moreover, branched or unbranched fin rays can coexist within one fin [110]. We observed the presence of second order bifurcation of fin rays in both second dorsal and caudal fins. This condition was found in the caudal lepidotrichia of the zebrafish [111] and in the pectoral fin rays ofsome batoids [112]. Fossil actinopterygians (e.g., Cheirolepis canadensis) [113] and sarcopterygians [114] are also known to possess fin rays with second to fifth order of bifurcation. Only Bendix-AIgrnreen [115] noticed first order bifurcation of radiais in paired fins of Cladoselache and other forms of bradyodonte. However, as far as we know, there is no case of second order bifurcation in radiais. Considering their position within the fins, the presence of both first and second order of bifurcation and their peculiar histologic morphology, the endochondral elements of P. marinus do possess the characteristics of true rays rather than fin radiais. The fin rays of P. marinus are not segmented and never mineralize, bringing their condition closer to that of the ceratotrichia. Another interesting feature of the fin ray of P. marinus is the absence of endoskeletal elements to connect to the vertebral column. The presence of a gap between the axial and the appendicular skeletons in selachians and Rajiformes is considered to be derived [32]; this condition is explained by the need to possess a fin with an independent action for a more active swimming mode of life. For now, we can only hypothesize that this condition in P. marinus may also be derived. The fin rays of P. marinus are the sole skeletal elements of the appendicular skeleton and develop proximodistally in order to reach the margin of the fin; this developmental pattern is consistent with the development of lepidotrichia of actinopterygians and dipnoans [51,98,103]. In addition, the proximal ends of the caudal fin rays lengthen anteriorly, in parallel to the neural tube; these extremities eventually fuse altogether to form the dorsomedian and ventromedian rods. Even though the appendicular skeleton of P. marinus is extremely simple, the developmental pattern of its fin rays is consistent with the one found in derived taxa. Thus, proximodistal development of rays is a very conservative pattern and likely represents a plesiomorphic condition for vertebrates. We showed that the fin rays of P. marinus are composed of layers of fiat rectangular chondrocytes; the same morphology can be observed in the branchial arches [9,25]. On the other han d, median rods are formed by large, pentagonal cells; such cellular morphology was also found in the trabecula, subchordal bar and parachordals of ammocoetes [9]. Our results suggest that two different cellular morphologies are present among the skeletal elements of P. marinus, thereby supporting the hypothesis of Martin et al. [9]. Based on the cellular morphological differences, Martin et al. [9] suggested that « type 1 » cartilage, consisting of stacked rectangular cells and described as « soft » tissue by Parker [94], possess a support function for gills and other tissues [9]; and « type II » cartilage, formed by larger polygonal cells and called « hard » tissue by Parker [94], would represent another type of tissue with an unknown function. Based on these descriptions, fin rays can be considered as a « type 1 » cartilage, whereas the median rods correspond to « type II » cartilage. However, even if the cellular morphology underlines an obvious difference between these types of cartilage in P. marinus, histological analysis showed that the cellular morphology of the cartilage were mostly the same for fin rays and medi an rods. In both cases, the cartilage is formed by piled-up chondrocytes surrounded by a peripheral extracellular matrix – very thin in the case of the median rods. The chondrocytes in the median rods are slightly larger, mure glubular and less regularly stacked; this observation represents the only noticeable difference between the two cartilages; our results therefore showed a clear delineation between the cartilage of the median rods and the cartilage of fin rays.
Metamorphosis
We observed the modifications of the skeleton in two stages of metamorphosis (i.e., stage 4 and stage 5). Because the neurocranium is the first skeletal system to show signs of modification [7,17], it was expected that skeletal elements of this region would already be formed by stage 4. In fact, neurocranial and branchial elements were always weil developed in stage 4 and stage 5 specimens, indicating that the development of these structures occurred in earlier stages of metamorphosis. Developmental patterns depend on the acquisition of structures that will quickly fulfil the functional requirements imposed by environmental and behavioural constraints [103]. Our results suggest that the development of the neurocranial and branchial elements are prioritized since their development and transformation is achieved before the buccal elements. However, the development of the feeding structures is normally prioritized over respiratory structures in osteichthyans [84]. The pharynx of the lampreys is a multifunctional system that serves for the respiration and osmoregulation [117]; thus, the transformation of the branchial skeleton may represent a priority during metamorphosis. AIso, considering that there is a 4 to 10 month period of fasting between the onset of metamorphosis in ammocoetes and the beginning of feeding in adults [38,108], the development of the branchial skeleton is more relevant. In addition,because the buccal skeleton is not initially present in the ammocoete, the time required for its development may be longer. The sequence of formation of buccal elements varies in terms of their relative timing. More specifically, four elements (i.e., anterior dorsal, posterior dorsal, anterior lateral, posterior lateral) did not develop according to the same sequence in stage 4 and stage 5. Our results suggest a lack of developmental coordination among buccal elements and also among skeletal systems in P. marinus. In fact, a metamorphosing ammocoete can possess a large number of well-developed arcualia in addition to epitrematic and hypotrematic protuberances present along each of the branchial arches, whereas the pericardial cartilage is underdeveloped. For other specimens, the pericardial cartilage is weil developed, but the epitrematic and hypotrematic protuberances are mostly absent. Specimens can even possess a well-developed pericardial cartilage, but few and weakly developed arcualia. Our observations strongly suggest that the skeletal systems (i.e., buccal, neurocranial, branchial, axial) develop independently in metamorphosing ammocoetes. As far as we know, asynchronous development among skeletal systems has never been documented in fishes. Ontogeny is a complex process that strongly depends on the timing of developmentalevents [118]. lt was previously assumed that the temporal sequence of developmental events is constant within a species [119]. However, minor intraspeci fic variation in ossification sequences has been documented in the skull (i .e., Danio rerio, Betta splendens) [79, 120] and the appendi cul ar skeleton (i.e., Salvelinus alpinus) [77,121] of some osteichthyans. Grünbaum et al. [77] specifically stated that intraspecific developmental vari ation occurs in terms of timing. [n fact, the timing of appearance and the timing of transitions of skeletal states (i.e., transition from cartilaginous to bony states) wou ld better explain the deve lopmental plasticity rather than the order of events within a sequence [77]. Thus, development can be variable intraspecifically and this variation mostly depends on the developmental timing. However, ail of these investigations refer to osteichthyans;observation of intraspecific variation has never been observed in a basal group such as lampreys. This suggests that the asynchronous development may be a plesiomorphic condition for vertebrates.
|
Table des matières
REMERCIEMENTS
TABLE DES MATIÈRES
LISTE DES TABLEAUX
LISTE DES FIGURES
LISTE DES ABRÉVIATIONS
INTRODUCTION GÉNÉRALE
CHAPITRE 1. ÉTUDE DE LA SQUELETTOGENÈSE ET DE LA MÉTAMORPHOSE CHEZ LA LAMPROIE MARINE (PETROMYZON MAR/NUS)
1.1 Résumé en fran çais du premier article
1.2 A study of the skeletogenesis and metamorphosis of the Sea Lamprey (Petromyzon marinus)
ABSTRACT
INTRODUCTION
MATERIAL AND METHODS
Specimens
Histology and Skeletal Preparation
Statistical Methods
Nomenclature and Definitions
RESULTS
AMMOCOETES
Skeletal Development
METAMORPHOSING AMMOCOETES
Staging
Skeletal Development
Morphology and Development of the Arcualia and the Mediodorsal Vertebral Elements
Fin Rays, Median Rods and Dorsolateral Cartilage
ADULTS
Skeletal Development
Morphology and Development of Arcualia and the Mediodorsal Vertebral Elements
Fin Rays, Median Rods ans Dorsolateral Cartilage
DISCUSSION
Patterning and Morphology of the Appendicular Skeleton
Fin Rays
Metamorphosis
Vertebral Elements, Mediodorsal Vertebral Elements and Notochordal Cartilage (Dorsolateral Cartilage)
Comparisons and Future « Vork
ACKNOWLEDGEMENTS
TABLES
FIGURES
ApPENDIX
LITERATURE CITED
CHAPITRE 2. CONCLUSION
RÉFÉRENCES BIBLIOGRAPHIQUES
![]() Télécharger le rapport complet
Télécharger le rapport complet