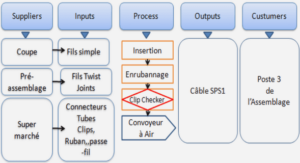
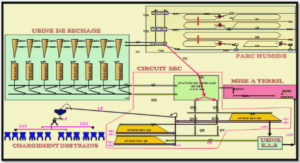
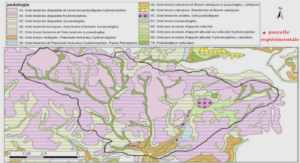
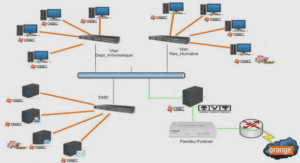
Anode Properties
Anodes are used in electrolytic cell as the carbon source for aluminum production. Carbon reacts with oxygen coming from the electrolytic reaction; as a result of this reaction, aluminum and CO2 are produced. Quality of anode directly affects the quality of resulting aluminum, aluminum production cost, and the amount of greenhouse gases emitted.
In aluminum production, anode manufacturing is one of the most important steps in order to obtain good quality aluminum. There are four major steps to produce anodes: preparation of raw materials, mixing, compaction, and anode baking . Each step has a significant effect on anode quality. Carbon anode contains calcined petroleum coke, coal tar pitch, and recycled materials (anode butts and green and baked rejects). Calcined petroleum coke (60-70%) and recycled materials (15-20%) are crushed to attain certain granulometry. These particles are called ‘dry aggregate’. The preparation of dry aggregate has a great impact on packing . Good packing is the first step for good quality anode. The anode recipe is prepared using different fractions of different size coke particles. The medium size particles should fill the void space between the coarse particles, and the space between medium particles should be filled with fine particles.
Fine particles (less than 1 mm) fill in the remaining space. If a large amount of fine particles are used in the recipe, the surface area of the recipe will be high. Higher surface area increases the required amount of pitch. Excess pitch causes more volatiles during baking, and it is costly.
Density
The high density is a desired property for a good quality anode. The density is related to anode life. Denser anodes provide longer anode life. The density of green anode is around 1.55-1.65 g/cm3 and the density of baked anode is 1.50-1.60 g/cm3. The density of anode is determined by dividing the weight by its volume.
The green density should be high to produce denser baked anodes. Raw materials quality, mixing conditions (mixing temperature and mixing time), and forming conditions (compaction time and frequency of compaction) have a significant effect on the green density. The low green density means more porosity, which can create cracks during baking. The baked density also should be high. If the baked density is low, the electrical resistivity is high because of porosity. On the other hand, too high a baked anode density could cause thermal shock.
Electrical Resistivity
The electrical resistivity of anode is related to energy consumption. Decrease in electrical resistivity reduces the energy consumption (and cost) during aluminum production.
Electrical resistivity depends on the coke properties, the density of anode, and the pores/cracks inside the anode. Generally, denser anodes have lower electrical resistivity since they have less porosity. The electrical resistivity of anode is around 50-60 µΩm. The green electrical resistivity is not usually measured, but it is known that the electrical resistivity of green anode is much higher than the electrical resistivity of baked anode.
High electrical resistivity causes more energy consumption. On the other hand, low electrical resistivity means high thermal conductivity. If the thermal conductivity of anode is high, the top temperature of anode increases more and this can cause air burn problems because carbon reacts more with air at higher temperatures.
Raw Materials Properties
Carbon anodes consist of approximately 60-70 % calcined petroleum coke, 20 % recycled anodes and butts, and 15 % coal tar pitch. The properties of these raw materials have a significant effect on resulting anode properties such as density, electrical resistivity, air and CO2 reactivities, mechanical properties and anode consumption.
Petroleum Coke
Green petroleum coke is a by-product of petroleum refinery, which constitutes about 2% of the overall production . Mechanical and physical properties of petroleum coke are dependent on the source of crude oil, processes within the refineries and the coke calcination conditions . The crude petroleum contains four different groups, namely aliphatic chains (low carbon), napthene (medium carbon), aromatic chains (high carbon), and the mixture. Petroleum cokes obtained from asphaltenes feedstock, which contains higher amount of aromatic chains and impurities, such as sulfur and metals (vanadium, nickel, calcium and sodium).
Green petroleum coke is calcined at a certain temperature (1200- 1300°C) which is usually higher than the anode baking temperature before using it in carbon anodes as one of the main raw materials . There are several reasons for calcination such as increasing C/H ratio, removing water and volatiles, decreasing impurities, minimizing the shrinkage during anode baking, and increasing coke strength. Calcination of coke ensures removing moisture and volatile matter from the coke. Therefore, it decreases cracking during the baking of anodes. Also, it helps pitch enter into the pores of coke during mixing stage in anode production.
Coal Tar Pitch
Coal tar pitch is used as the binder in order to create a bond between dry aggregates. It is defined by the International Committee for Characterization and Terminology of Carbon as a residue produced by distillation or heat treatment of coal tar. The physical and chemical properties of coal tar pitch are dependent on the processes conditions and the source of tar. It has a complex structure, which contains aromatic and heteroatom-containing functional groups . During anode baking, pitch carbonizes and binds with the complimentary functional groups of coke. The wetting properties of coal tar pitch affect the resulting anode properties. Good wetting properties of coke by pitch means that pitch not only penetrates into the pores of calcined coke but also fills the void space between the particles.
There are some important properties of pitch, which influence the anode properties such as softening point, density, coking value, and impurities. The softening point of pitch varies. Higher softening point pitches usually help produce denser anodes. Coking value of pitch increases with increasing softening point. In order to obtain good anode properties, pitch should have reasonable coking value (54-61) . The density of pitch is 1.30- 1.32 g/cm3 . Better quality anodes can be produced with increasing pitch density, which provides higher aromaticity. Binder pitch should have higher aromaticity to create bonds between coke particles. The impurities in pitch such as sulfur, sodium, calcium, nickel, and vanadium affect the anode reactivity.
Wettability of Coke by Pitch
Wettability of a solid surface by a liquid is the function of the surface and interfacial forces which are both adhesive and cohesive (physical wetting) and the chemical interactions (chemical wetting). Thus, the wettability of coke by pitch can be not only physical due to adhesive and cohesive forces (intermolecular interactions), but also chemical because of the interface reactions between coke and pitch. The spontaneous interaction is observed when the molten pitch contacts the coke surface. This pattern is used to determine the wetting properties of coke. Wettability of coke by pitch determines the quality of interaction between these two components. Better interaction between these raw materials during mixing directly affects the resulting anode properties such as density, electrical resistivity, air and CO2 reactivities, and mechanical properties .
Improved wettability of coke by pitch helps pitch better penetrate into the pores of the coke as well as the void between different particles. The interactions between the pitch and the particles depend on a number of properties of the latter, notably the particle size, the texture, and the chemical functional groups on the surface.
The wettability of coke by pitch can be quantified by the contact angle. Contact angle is the angle between the solid surface (coke) and the liquid (pitch) when the liquid drops on the solid surface . The relationship between the interfacial tensions and the contact angle is expressed by the Young equation . In the equation, γLV is the interfacial tension of the liquid-vapor interface, γSV is the interfacial tension of the solid- vapor interface, γLS is the liquid-solid interface, and θ is the contact angle. The interfacial tension of the liquid-vapor interface is also called the surface tension (γLV). The force balance given by the Young’s equation is applied at the triple point (contact point of solid, liquid, and vapor phases).
Fourier Transform Infrared Spectroscopy (FT-IR)
Coke and pitch are the main components for anode production, and their quality of can directly affect the properties of anode. Both components have complex structures to analyze. The chemical structure of coke and pitch plays an important role in the interaction between coke and pitch at the mixing stage during anode manufacturing. FT-IR is one of the most common, easy, and inexpensive techniques to analyze the structure of materials. It gives an idea about presence of the functional groups, which help coke-pitch interaction, in coke, pitch, and additives. In this study, coke, pitch and additives were analyzed by using Nicolet 6700. Characterization was done using KBr, and the ratio of KBr to sample was around 200:1. The number of scans was 36, and the spectra were registered for the wavenumber range of 400-4000 cm-1. Each experiment was repeated two times, and the average value was used for the analysis. Linear baseline correction was carried out for each sample using the Omnic software.
|
Table des matières
CHAPTER 1 :INTRODUCTION
1.1. Background
1.2. Statement of Problem
1.3. Objectives
1.4. Methodology
1.5. Scope
CHAPTER 2 :LITERATURE REVIEW
2.1. Anode Properties
2.1.1. Density
2.1.2. Electrical Resistivity
2.1.3. Air/CO2 Reactivity
2.1.4. Mechanical Properties
2.2. Raw Materials Properties
2.2.1. Petroleum Coke
2.2.2. Coal Tar Pitch
2.2.3. Anode Butts
2.3. Wettability of Coke by Pitch
2.3.1. Modification of Pitch
2.3.2. Modification of Coke
CHAPTER 3 :EXPERIMENTAL
3.1. Characterization of Raw Materials
3.1.1. Modification of Coke with Additives for Characterizations
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.1.3. Wettability
3.1.4. Tapped Bulk Density
3.2. Preparation of Laboratory Scale Anodes
3.2.1. Dry Aggregate Preparation
3.2.2. Modification of Dry Aggregate with an Additive for Anode Production
3.2.3. Mixing
3.2.4. Vibrocompaction
3.2.5. Baking
3.3. Characterization of Laboratory Scale Anodes
3.3.1. Sample Preparation
3.3.2. Apparent Density
3.3.3. Electrical Resistivity
3.3.4. Air and CO2 Reactivities
3.3.5. Flexural Strength
CHAPTER 4 :MODIFICATION OF COKE BY DIFFERENT ADDITIVES TO IMPROVE ANODE PROPERTIES
4.1. Introduction
4.2. Experimental
4.2.1. Material Used
4.2.2. Modification of Calcined Coke with Additive
4.2.3. Sample Characterization
4.2.4. Fabrication of Laboratory Anodes
4.2.5. Characterization of Anode Properties
4.3. Results and Discussions
4.3.1. FT-IR Results
4.3.2. Wettability
4.3.3. Effects of Additive on Anode Properties
4.4. Conclusions
CHAPTER 5 :MODIFICATION OF PETROLEUM COKE BY DIFFERENT ADDITIVES AND THE
IMPACT ON ANODE PROPERTIES
5.1. Introduction
5.2. Experimental
5.2.1. Material Used
5.2.2. Modification of Calcined Coke/Aggregate with Additive
5.2.3. Sample Characterization
5.2.4. Fabrication of Laboratory Anodes
5.2.5. Characterization of Anode Properties
5.3. Results and Discussions
5.3.1. FT-IR Results
5.3.2. Wettability Results
5.3.3. Effects of Additive on Anode Properties
5.4. Conclusions
CHAPTER 6 :EFFECT OF THE ADDITIVE CONCENTRATION ON THE MODIFICATION OF PETROLEUM COKE AND ANODE QUALITY
6.1. Introduction
6.2. Experimental
6.2.1. Material Used
6.2.2. Modification of Calcined Coke with Additive
6.2.3. Sample Characterization
6.2.4. Fabrication of Laboratory Anodes
6.2.5. Characterization of Anode Properties
6.3. Results and Discussions
6.3.1. FT-IR Results
6.3.2. Wettability Results
6.3.3. Effects of Additive on Anode Properties
6.4. Conclusions
CHAPTER 7 :CONCLUSIONS AND RECOMMENDATIONS
7.1. Conclusions
7.2. Recommendations
REFERENCES