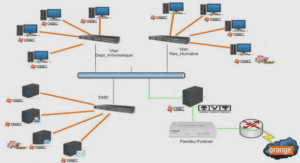
Comparaison of cytochrome c concentration and its effect on the maximal velocity
Animal and chemical supply
Two species of ectotherms and two species of endotherms were used for this experiment. Specimens of N. virens were captured in March 2003 in Sainte-Luce sur Mer, Québec. Arctic charr were bought from the Les Cèdres fishfarm, Luceville. Rabbits were bought from a local farm and specimens of honeybees were obtained from a local bee-keeper; les Productions du Vieux Moulin, Sainte-Luce sur Mer in July 2003.
Chemicals were bought from Sigma-Aldrich corporation.
COX extraction
Ali extractions were done with fresh tissue. For N. virens and honeybees, individuals have been pooled to obtain enough purified enzymes to allow ail essays. A total of about 25 and 100 individuals for each extraction were respectively pooled prior to homogeneization.
Four extractions were done on each species. For arctic char, both lateral-red muscles were removed and pooled. Muscles from the rear-upper leg were removed and pooled for rabbits.
Once removed, tissues were homogenized in 4 volumes of an homogeneization buffer (250 mM sucrose; 1 mM EDT A; 30 mM HEPES; 1 % (w/v) bovine albumin serum and 0,1% of tween 20; pH 7,4) using a tekmar polytron. Ali procedures were performed on ice, except the centrifugations which were done at 4° C. The homogenate was then centrifuged (Heraeus Sepatech; suprafuge 22) at 750g for 10 minutes and the supernatant, without the layer of lipid at the surface, was removed and kept at 4D C until further use. The pellet was resuspended in 3 volumes of homogeneization buffer and re-homogenized with a tekmar polytron. A second centrifugation at 750g x 10 min was done. The supernatant was then removed and mixed with the previous one. They were then centrifuged at 27 OOOg for 10 minutes and the supernatant, containing the cytochrome c, was discarded. The pellet was resuspended in 3 volumes of Tris-HCl buffer (10 mM; pH = 7,4) containing 0,1 % oftween20. A second centrifugation of 27 OOOg x 10 minutes was then done. The pellet was then resuspended in 4 volumes of HEPES-KOH buffer (100mM; pH = 7,4) and frozen in 500ul aliquots in liquid nitrogen.
Respiration measurements
Activity of COX was measured in a polarographic system, using an oxymeter (Cameron Instrument Co., Tx, USA), two Clark-type O2 electrodes and two water-jacketed glass chambers. The system was calibrated using distilled water; 637 nmol O2 mr 1 at IODC, 521 nmol 02 mr 1 at 20DC and 444 nmol O2 mr 1 at 30DC (Reynafarje et al., 1985). The assays were conducted in 130 mM KC1, 30 mM HEPES, 10 mM KH2P04, Il mM MgCh, 20 mM glucose, 0,5% BSA and 10mM ascorbic acid pH 7,4 (Blier and Lemieux, 2001). Residual activity was measured for 3 minutes and the respiration was initiated by the addition of CYC to reach desired concentration every 2 minutes. Respiration rates were determined by increasing the concentration of CYC in the reactive chambers, ranging from 2 to 480 /lM of CYC. A first reaction was monitored for low concentrations of CYC, from 2 to 30 )lM.
The same protocol was used subsequently, for higher concentrations of CYC (30 to 480)lM). This was used to maximize the readability of the activity at low concentrations of CYC. AlI assays were done on four extractions for each species. Under these conditions, reaction rates for each concentration were linear and were calculated using the best linear fit to the 1,5 min trajectories. Reaction rates were normalized to protein concentration of the homogenates as determined with bicinchoninic acid reagent (BSA, Sigma) with BSA standards (Smith et al., 1985).
Citrate synthase activity
The activity of the citrate synthase was measured at the three experimental temperatures using the method of Thibault et al., (1997). Samples were unfrozen and diluted to experimental concentration with HEPES-KOH (100 mM; pH=7,4). Essays were conducted in an imidazole-HCI (100mM, pH=8,0) in presence of 0,1 mM of DTNB, 0,1 mM of actylcoenzyme A and 0, 15mM of oxaloacetic acid. Activity was monitored for three minutes and reaction rates were normalized to protein conc~ntration of the homogenates as determined with bicinchoninic acid reagent (BSA, Sigma) with BSA standards (Smith et al., 1985). Citrate synthase (CS) is a good marker of mitochondrial volume in tissues and we used this activity measured at 20°C as an indicator of mitochondrial content to
normalize COX activity.
COX thermal stability
The thermal stability on the COX was measured on three extractions per species with CYC from horse heart. The homogenates were diluted with HEPES-KOH buffer (100 mM, pH=7,4) and incubated at 30°C for 36 hours. The activity of COX was measured polarographicalIy at 0, 1, 2, 4, 8, 12, 24 and 36 hours of incubation. The assays were conducted in the same conditions used to determine the Km and Vmax and CYC at a concentration of 5 times the determined Km in polarography. Reaction rates were normalized to protein concentration of the homogenates as determined with bicinchoninic acid reagent (BSA, Sigma) with BSA standards (Smith et al., 1985). The activity was first measured on freshly unfrozen samples and the activity obtained was considered as a reference. AlI subsequent activities were divided by this one to obtain the percentage of initial activity.
Comparison of cytochrome c concentration and its effect on the maximal velo city
To compare the maximal velocities obtained with those from other studies, we calculated velocities at published CYC concentration, using the Km and Vmax from the present study.
The concentrations of CYC chosen for the calculation are commonly used in the literature when COX activity is measured using a spectrophotometer (50 and 100/-!M). Generated activities were then divided by our Vmax values to give a magnitude of error associated with the low concentrations of substrate used in many previous studies.
Thermal stability of COX
The four species tested for thermal stability show very clear patterns. Rabbits maintained their initial activity after 24 hours of incubation at 30 D e. Honeybees lost activity much faster; aimost 50% within the first hour. Cold-adapted species were able to maintain activity longer than honeybees, but still lost activity faster than rabbits; almost 60% of the activity was lost within the 4 first hours for N virens and 25% for arctic charr for the same period. After two hour of incubation, rabbits showed a significantly higher relative activity than other species and that difference is found in all subsequent essays . These results suggest a high thermal stability of the rab bit enzyme compare to the other species, although we expected a good stability at 30 D C for honeybees.
|
Table des matières
Chapitre 1. Introduction
Chapitre 2
1. Abstract
2. Introduction
3. Material and Methods
Animal and chemical supply
COX extraction
Respiration measurements
Citrate synthase activity
COX thermal stability
Comparaison of cytochrome c concentration and its effect on the maximal velocity
Statistical analysis
Genetic analysis
4. Results
Km and maximal velocity variations in relation to the source ofCYC
Impact oftemperature on the Km and Vmax of COX
Interspecific variation of the Km and maximal velocity
QIO values
Thermal stability of COX
Genetic analysis
Comparaison of CYC concentration and its effect on the maximal velocity of COX
5. Discussion
Thermal stability at high tempe rature
Impact of temperature on COX
Comparaison ofmethods of COX activity measurements
6. Conclusion
7. Acknowledgements
Chapitre 3. Conclusion
Références bibilographiques
![]() Télécharger le rapport complet
Télécharger le rapport complet